Virus and Host Mechanics Support Membrane Penetration and Cell Entry
- PMID: 26842477
- PMCID: PMC4810568
- DOI: 10.1128/JVI.02568-15
Virus and Host Mechanics Support Membrane Penetration and Cell Entry
Abstract
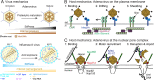
Viruses are quasi-inert macromolecular assemblies. Their metastable conformation changes during entry into cells, when chemical and mechanical host cues expose viral membrane-interacting proteins. This leads to membrane rupture or fusion and genome uncoating. Importantly, virions tune their physical properties and enhance penetration and uncoating. For example, influenza virus softens at low pH to uncoat. The stiffness and pressure of adenovirus control uncoating and membrane penetration. Virus and host mechanics thus present new opportunities for antiviral therapy.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

