Vessel Dilation Attenuates Endothelial Dysfunction Following Middle Cerebral Artery Occlusion in Hyperglycemic Rats
- PMID: 26842484
- PMCID: PMC6492847
- DOI: 10.1111/cns.12500
Vessel Dilation Attenuates Endothelial Dysfunction Following Middle Cerebral Artery Occlusion in Hyperglycemic Rats
Abstract
Objectives: Dynamically observe cerebral vascular changes in hyperglycemic rats in vivo and explore the effect of diabetes on endothelial function after ischemic stroke.
Background: Diabetes affects both large and small vessels in the brain, but the dynamic process and mechanism are unclear.
Methods: We investigated the structural and functional changes of brain vasculature in living hyperglycemic rats and their impact on stroke outcomes via a novel technique: synchrotron radiation angiography. We also examined the effect of prolonged fasudil treatment on arterial reactivity and hemorrhagic transformation. Adult Sprague Dawley rats were treated by streptozotocin to induce type 1 diabetes. These hyperglycemic rats received fasudil pretreatment and then underwent transient middle cerebral artery occlusion.
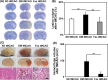
Results: We found that diabetes caused arteries narrowing in the circus Willis as early as 2 weeks after streptozotocin injection (P < 0.05). These vessels were further constricted after middle cerebral artery occlusion. L-NAME could induce regional constrictions and impaired relaxation in hyperglycemic animals. Furthermore, hemorrhagic transformation was also increased in the hyperglycemic rats compared to the control (P < 0.05). In fasudil-treated rats, the internal carotid artery narrowing was ameliorated and L-NAME-induced regional constriction was abolished. Importantly, stroke prognosis was improved in fasudil-treated rats compared to the control (P < 0.05).
Conclusions: Our dynamic angiographic data demonstrated that diabetes could impair the cerebral arterial reactivity. Prolonged fasudil treatment could attenuate arterial dysfunction and improve the prognosis of ischemic stroke by affecting both the large and small vasculature.
Keywords: Fasudil; Hyperglycemia; Ischemia; Synchrotron radiation; Vascular dysfunction.
© 2016 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation 1999;100:1134–1146. - PubMed
-
- Alvarez‐Sabin J, Molina CA, Montaner J, et al. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator–treated patients. Stroke 2003;34:1235–1241. - PubMed
-
- Cosentino F, Rubattu S, Savoia C, Venturelli V, Pagannonne E, Volpe M. Endothelial dysfunction and stroke. J Cardiovasc Pharmacol 2001;38(Suppl 2):S75–S78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

