Phylogeny and adaptation shape the teeth of insular mice
- PMID: 26842576
- PMCID: PMC4760175
- DOI: 10.1098/rspb.2015.2820
Phylogeny and adaptation shape the teeth of insular mice
Abstract
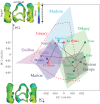
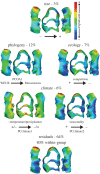
By accompanying human travels since prehistorical times, the house mouse dispersed widely throughout the world, and colonized many islands. The origin of the travellers determined the phylogenetic source of the insular mice, which encountered diverse ecological and environmental conditions on the various islands. Insular mice are thus an exceptional model to disentangle the relative role of phylogeny, ecology and climate in evolution. Molar shape is known to vary according to phylogeny and to respond to adaptation. Using for the first time a three-dimensional geometric morphometric approach, compared with a classical two-dimensional quantification, the relative effects of size variation, phylogeny, climate and ecology were investigated on molar shape diversity across a variety of islands. Phylogeny emerged as the factor of prime importance in shaping the molar. Changes in competition level, mostly driven by the presence or absence of the wood mouse on the different islands, appeared as the second most important effect. Climate and size differences accounted for slight shape variation. This evidences a balanced role of random differentiation related to history of colonization, and of adaptation possibly related to resource exploitation.
Keywords: Mus musculus domesticus; first upper molar; house mouse; insular evolution; three-dimensional geometric morphometrics.
© 2016 The Author(s).
Figures

References
-
- Berry RJ. 1973. Chance and change in British long-tailed field mice (Apodemus sylvaticus). J. Zool. 170, 351–366. (10.1111/j.1469-7998.1973.tb01383.x) - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
