Evaluation of Performance Characteristics of the Aptima HIV-1 Quant Dx Assay for Detection and Quantitation of Human Immunodeficiency Virus Type 1 in Plasma and Cervicovaginal Lavage Samples
- PMID: 26842702
- PMCID: PMC4809915
- DOI: 10.1128/JCM.03289-15
Evaluation of Performance Characteristics of the Aptima HIV-1 Quant Dx Assay for Detection and Quantitation of Human Immunodeficiency Virus Type 1 in Plasma and Cervicovaginal Lavage Samples
Abstract
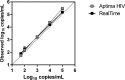
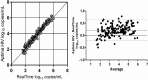
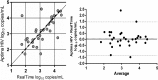
Quantification of HIV-1 RNA has become the standard of care in the clinical management of HIV-1-infected individuals. The objective of this study was to evaluate performance characteristics and relative workflow of the Aptima HIV-1 Quant Dx assay in comparison with the Abbott RealTime HIV-1 assay using plasma and cervicovaginal lavage (CVL) specimens. Assay performance was evaluated by using an AcroMetrix HIV-1 panel, AcroMetrix positive controls, Qnostics and SeraCare HIV-1 evaluation panels, 208 clinical plasma samples, and 205 matched CVL specimens on the Panther and m2000 platforms. The Aptima assay demonstrated good linearity over the quantification range tested (2 to 5 log10copies/ml), and there was strong linear correlation between the assays (R(2)= 0.99), with a comparable coefficient of variance of <5.5%. For the plasma samples, Deming regression analyses and Bland-Altman plots showed excellent agreement between the assays, with an interassay concordance of 91.35% (kappa = 0.75; 95% confidence interval [CI], 0.65 to 0.85), and on average, the viral loads determined by the Aptima assay were 0.21 log10copies/ml higher than those determined by the RealTime assay. The assays differed in their sensitivity for quantifying HIV-1 RNA loads in CVL samples, with the Aptima and RealTime assays detecting 30% and 20%, respectively. Aptima had fewer invalid results, and on average, the viral loads in CVL samples quantified by the Aptima assay were 0.072 log10copies/ml higher than those of the RealTime assay. Our results demonstrate that the Aptima assay is sensitive and accurate in quantifying viral loads in both plasma and CVL specimens and that the fully automated Panther system has all the necessary features suitable for clinical laboratories demanding high-throughput sample processing.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- CDC. 2015. Sexually transmitted diseases treatment guidelines. MMWR Morb Mortal Wkly Rep 64:1–137. - PubMed
-
- Hart CE, Lennox JL, Pratt-Palmore M, Wright TC, Schinazi RF, Evans-Strickfaden T, Bush TJ, Schnell C, Conley LJ, Clancy KA, Ellerbrock TV. 1999. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis 179:871–882. doi:10.1086/314656. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

