ABCC4 Is a Determinant of Cytarabine-Induced Cytotoxicity and Myelosuppression
- PMID: 26842729
- PMCID: PMC4905720
- DOI: 10.1111/cts.12366
ABCC4 Is a Determinant of Cytarabine-Induced Cytotoxicity and Myelosuppression
Abstract
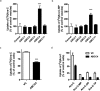
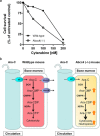
Resistance to cytarabine remains a major challenge in the treatment of acute myeloid leukemia (AML). Based on previous studies implicating ABCC4/MRP4 in the transport of nucleosides, we hypothesized that cytarabine is sensitive to ABCC4-mediated efflux, thereby decreasing its cytotoxic response against AML blasts. The uptake of cytarabine and its monophosphate metabolite was found to be facilitated in ABCC4-expressing vesicles and intracellular retention was significantly impaired by overexpression of human ABCC4 or mouse Abcc4 (P < 0.05). ABCC4 was expressed highly in AML primary blasts and cell lines, and cytotoxicity of cytarabine in cells was increased in the presence of the ABCC4 inhibitors MK571 or sorafenib, as well as after ABCC4 siRNA. In Abcc4-null mice, cytarabine-induced hematological toxicity was enhanced and ex vivo colony-forming assays showed that Abcc4-deficiency sensitized myeloid progenitors to cytarabine. Collectively, these studies demonstrate that ABCC4 plays a protective role against cytarabine-mediated insults in leukemic and host myeloid cells.
© 2016 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures



References
-
- Borst, P. , de Wolf, C. & van de Wetering, K. Multidrug resistance‐associated proteins 3, 4, and 5. Pflugers Arch. 453, 661–673 (2007). - PubMed
-
- Peng, X.X. et al Up‐regulation of MRP4 and down‐regulation of influx transporters in human leukemic cells with acquired resistance to 6‐mercaptopurine. Leuk. Res. 32, 799–809 (2008). - PubMed
-
- Liu, B. , Zhao, L. , Ma, H. , Zhang, W. & Jin, Y. Knockdown of MRP4 by lentivirus‐mediated siRNA improves sensitivity to adriamycin in adriamycin‐resistant acute myeloid leukemia cells. Chin. Sci. Bull. 57, 90–97 (2012).
-
- Oevermann, L. et al Hematopoietic stem cell differentiation affects expression and function of MRP4 (ABCC4), a transport protein for signaling molecules and drugs. Int. J. Cancer 124, 2303–2311 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

