Benefits of E-Cigarettes Among Heavy Smokers Undergoing a Lung Cancer Screening Program: Randomized Controlled Trial Protocol
- PMID: 26842790
- PMCID: PMC4757781
- DOI: 10.2196/resprot.4805
Benefits of E-Cigarettes Among Heavy Smokers Undergoing a Lung Cancer Screening Program: Randomized Controlled Trial Protocol
Abstract
Background: Smoking is a global public health problem. For this reason, experts have called smoking dependence a global epidemic. Over the past 5 years, sales of electronic cigarettes, or e-cigarettes, have been growing strongly in many countries. Yet there is only partial evidence that e-cigarettes are beneficial for smoking cessation. In particular, although it has been proven that nicotine replacement devices may help individuals stop smoking and tolerate withdrawal symptoms, e-cigarettes' power to increase the quitting success rate is still limited, ranging from 5% to 20% dependent on smokers' baseline conditions as shown by a recent Cochrane review. Consequently, it is urgent to know if e-cigarettes may have a higher success rate than other nicotine replacement methods and under what conditions. Furthermore, the effects of the therapeutic setting and the relationship between individual characteristics and the success rate have not been tested. This protocol is particularly innovative, because it aims to test the effectiveness of electronic devices in a screening program (the COSMOS II lung cancer prevention program at the European Institute of Oncology), where tobacco reduction is needed to lower individuals' lung cancer risks.
Objective: This protocol was designed with the primary aim of investigating the role of tobacco-free cigarettes in helping smokers improve lung health and either quit smoking or reduce their tobacco consumption. In particular, we aim to investigate the impact of a 3-month e-cigarettes program to reduce smoking-related respiratory symptoms (eg, dry cough, shortness of breath, mouth irritation, and phlegm) through reduced consumption of tobacco cigarettes. Furthermore, we evaluate the behavioral and psychological (eg, well-being, mood, and quality of life) effects of the treatment.
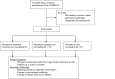
Methods: This is a prospective, randomized, placebo-controlled, double-blind, three-parallel group study. The study is organized as a nested randomized controlled study with 3 branches: a nicotine e-cigarettes group, a nicotine-free e-cigarettes group, and a control group. The study is nested in a screening program for early lung cancer detection in heavy smokers.
Results: The study is open and is still recruiting.
Conclusions: Stopping or reducing tobacco consumption should be a main goal of any health organization. However, traditional antismoking programs are expensive and not always effective. Therefore, favoring a partial or complete shift to e-cigarettes in heavy smokers (eg, persons at high risk for a number of diseases) could be considered a moral imperative. However, before following this path, sound and reliable data on large samples and in a variety of contexts are required.
Trial registration: Clinicaltrials.gov NCT02422914; https://clinicaltrials.gov/ct2/show/NCT02422914 (Archived by WebCite at http://www.webcitation.org/6etwz1bPL).
Keywords: electronic cigarettes; lung cancer screening; smoking related diseases.; tobacco cessation.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014 Apr;5(2):67–86. doi: 10.1177/2042098614524430. http://europepmc.org/abstract/MED/25083263 - DOI - PMC - PubMed
-
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317. http://dx.plos.org/10.1371/journal.pone.0066317 - DOI - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous