RNase P protein subunit Rpp29 represses histone H3.3 nucleosome deposition
- PMID: 26842893
- PMCID: PMC4814222
- DOI: 10.1091/mbc.E15-02-0099
RNase P protein subunit Rpp29 represses histone H3.3 nucleosome deposition
Abstract
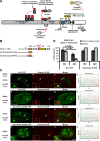
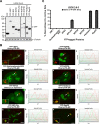
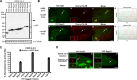
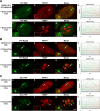
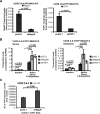
In mammals, histone H3.3 is a critical regulator of transcription state change and heritability at both euchromatin and heterochromatin. The H3.3-specific chaperone, DAXX, together with the chromatin-remodeling factor, ATRX, regulates H3.3 deposition and transcriptional silencing at repetitive DNA, including pericentromeres and telomeres. However, the events that precede H3.3 nucleosome incorporation have not been fully elucidated. We previously showed that the DAXX-ATRX-H3.3 pathway regulates a multi-copy array of an inducible transgene that can be visualized in single living cells. When this pathway is impaired, the array can be robustly activated. H3.3 is strongly recruited to the site during activation where it accumulates in a complex with transcribed sense and antisense RNA, which is distinct from the DNA/chromatin. This suggests that transcriptional events regulate H3.3 recruited to its incorporation sites. Here we report that the nucleolar RNA proteins Rpp29, fibrillarin, and RPL23a are also components of this H3.3/RNA complex. Rpp29 is a protein subunit of RNase P. Of the other subunits, POP1 and Rpp21 are similarly recruited suggesting that a variant of RNase P regulates H3.3 chromatin assembly. Rpp29 knockdown increases H3.3 chromatin incorporation, which suggests that Rpp29 represses H3.3 nucleosome deposition, a finding with implications for epigenetic regulation.
© 2016 Newhart et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures




Similar articles
-
Rpp29 regulates histone H3.3 chromatin assembly through transcriptional mechanisms.J Biol Chem. 2018 Aug 10;293(32):12360-12377. doi: 10.1074/jbc.RA118.001845. Epub 2018 Jun 19. J Biol Chem. 2018. PMID: 29921582 Free PMC article.
-
Single cell analysis of RNA-mediated histone H3.3 recruitment to a cytomegalovirus promoter-regulated transcription site.J Biol Chem. 2013 Jul 5;288(27):19882-99. doi: 10.1074/jbc.M113.473181. Epub 2013 May 20. J Biol Chem. 2013. PMID: 23689370 Free PMC article.
-
PML is recruited to heterochromatin during S phase and represses DAXX-mediated histone H3.3 chromatin assembly.J Cell Sci. 2019 Mar 26;132(6):jcs220970. doi: 10.1242/jcs.220970. J Cell Sci. 2019. PMID: 30796101 Free PMC article.
-
New players in heterochromatin silencing: histone variant H3.3 and the ATRX/DAXX chaperone.Nucleic Acids Res. 2016 Feb 29;44(4):1496-501. doi: 10.1093/nar/gkw012. Epub 2016 Jan 14. Nucleic Acids Res. 2016. PMID: 26773061 Free PMC article. Review.
-
[Histone variants and histone exchange].Yi Chuan. 2006 Apr;28(4):493-500. Yi Chuan. 2006. PMID: 16606605 Review. Chinese.
Cited by
-
The rice RNase P protein subunit Rpp30 confers broad-spectrum resistance to fungal and bacterial pathogens.Plant Biotechnol J. 2021 Oct;19(10):1988-1999. doi: 10.1111/pbi.13612. Epub 2021 May 17. Plant Biotechnol J. 2021. PMID: 33932077 Free PMC article.
-
The many faces of RNA-based RNase P, an RNA-world relic.Trends Biochem Sci. 2021 Dec;46(12):976-991. doi: 10.1016/j.tibs.2021.07.005. Epub 2021 Sep 9. Trends Biochem Sci. 2021. PMID: 34511335 Free PMC article. Review.
-
Two Novel Candidate Genes for Insulin Secretion Identified by Comparative Genomics of Multiple Backcross Mouse Populations.Genetics. 2018 Dec;210(4):1527-1542. doi: 10.1534/genetics.118.301578. Epub 2018 Oct 19. Genetics. 2018. PMID: 30341086 Free PMC article.
-
Decoding the ribosome's hidden language: rRNA modifications as key players in cancer dynamics and targeted therapies.Clin Transl Med. 2024 May;14(5):e1705. doi: 10.1002/ctm2.1705. Clin Transl Med. 2024. PMID: 38797935 Free PMC article. Review.
-
The Diversity of Ribonuclease P: Protein and RNA Catalysts with Analogous Biological Functions.Biomolecules. 2016 May 13;6(2):27. doi: 10.3390/biom6020027. Biomolecules. 2016. PMID: 27187488 Free PMC article. Review.
References
-
- Bender S, Tang Y, Lindroth AM, Hovestadt V, Jones DT, Kool M, Zapatka M, Northcott PA, Sturm D, Wang W, et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell. 2013;24:660–672. - PubMed
-
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

