Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature
- PMID: 26843061
- PMCID: PMC4740994
- DOI: 10.1186/s12916-016-0553-2
Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature
Erratum in
-
Correction to: Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature.BMC Med. 2019 Mar 2;17(1):56. doi: 10.1186/s12916-019-1294-9. BMC Med. 2019. PMID: 30823879 Free PMC article.
Abstract
Background: There have been no studies of the patterns of post-marketing withdrawals of medicinal products to which adverse reactions have been attributed. We identified medicinal products that were withdrawn because of adverse drug reactions, examined the evidence to support such withdrawals, and explored the pattern of withdrawals across countries.
Methods: We searched PubMed, Google Scholar, the WHO's database of drugs, the websites of drug regulatory authorities, and textbooks. We included medicinal products withdrawn between 1950 and 2014 and assessed the levels of evidence used in making withdrawal decisions using the criteria of the Oxford Centre for Evidence Based Medicine.
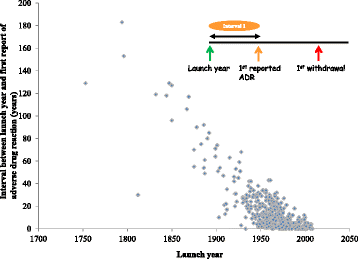
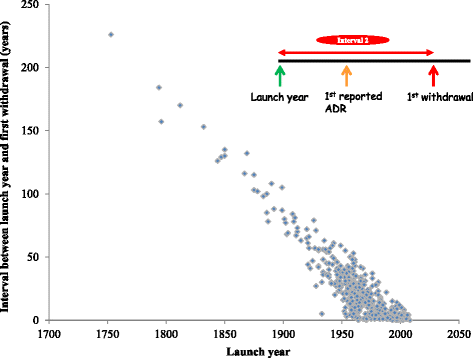
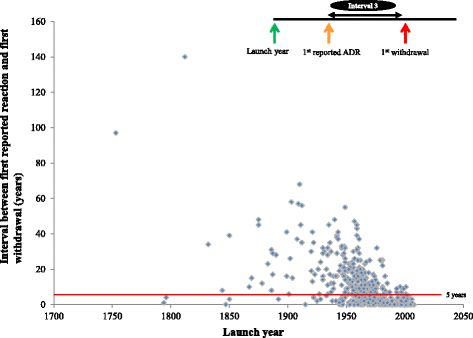
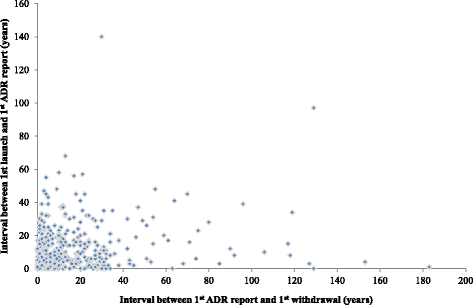
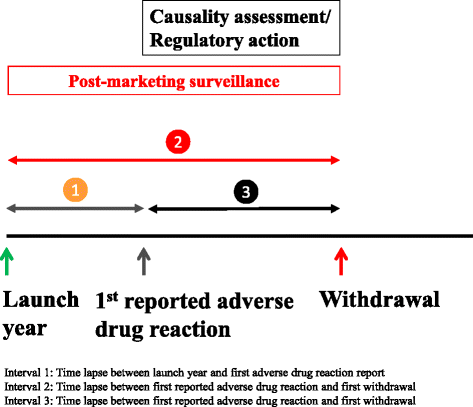
Results: We identified 462 medicinal products that were withdrawn from the market between 1953 and 2013, the most common reason being hepatotoxicity. The supporting evidence in 72 % of cases consisted of anecdotal reports. Only 43 (9.34 %) drugs were withdrawn worldwide and 179 (39 %) were withdrawn in one country only. Withdrawal was significantly less likely in Africa than in other continents (Europe, the Americas, Asia, and Australasia and Oceania). The median interval between the first reported adverse reaction and the year of first withdrawal was 6 years (IQR, 1-15) and the interval did not consistently shorten over time.
Conclusion: There are discrepancies in the patterns of withdrawal of medicinal products from the market when adverse reactions are suspected, and withdrawals are inconsistent across countries. Greater co-ordination among drug regulatory authorities and increased transparency in reporting suspected adverse drug reactions would help improve current decision-making processes.
Figures

Comment in
-
Drug safety: withdrawn medications are only part of the picture.BMC Med. 2016 Feb 13;14:28. doi: 10.1186/s12916-016-0579-5. BMC Med. 2016. PMID: 26873482 Free PMC article.
References
-
- Furberg CD. Understanding drug safety and how to maximize it for patients. JAAPA. 2011;24:16. - PubMed
-
- Sullivan JW. A pharmaceutical manufacturer’s perspective on reporting adverse drug experiences. Am J Hosp Pharm. 1990;47:1342–5. - PubMed
-
- Anonymous New contraindication added to Reclast drug label. Reactions Weekly. 2011;1368:3.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

