Evidence for Noncanonical Neurotransmitter Activation: Norepinephrine as a Dopamine D2-Like Receptor Agonist
- PMID: 26843180
- PMCID: PMC4809307
- DOI: 10.1124/mol.115.101808
Evidence for Noncanonical Neurotransmitter Activation: Norepinephrine as a Dopamine D2-Like Receptor Agonist
Abstract
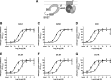
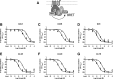
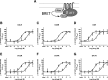
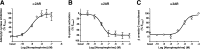
The Gαi/o-coupled dopamine D2-like receptor family comprises three subtypes: the D2 receptor (D2R), with short and long isoform variants (D2SR and D2LR), D3 receptor (D3R), and D4 receptor (D4R), with several polymorphic variants. The common overlap of norepinephrine innervation and D2-like receptor expression patterns prompts the question of a possible noncanonical action by norepinephrine. In fact, previous studies have suggested that norepinephrine can functionally interact with D4R. To our knowledge, significant interactions between norepinephrine and D2R or D3R receptors have not been demonstrated. By using radioligand binding and bioluminescent resonance energy transfer (BRET) assays in transfected cells, the present study attempted a careful comparison between dopamine and norepinephrine in their possible activation of all D2-like receptors, including the two D2R isoforms and the most common D4R polymorphic variants. Functional BRET assays included activation of G proteins with all Gαi/o subunits, adenylyl cyclase inhibition, and β arrestin recruitment. Norepinephrine acted as a potent agonist for all D2-like receptor subtypes, with the general rank order of potency of D3R > D4R ≥ D2SR ≥ D2L. However, for both dopamine and norepinephrine, differences depended on the Gαi/o protein subunit involved. The most striking differences were observed with Gαi2, where the rank order of potencies for both dopamine and norepinephrine were D4R > D2SR = D2LR >> D3R. Furthermore the results do not support the existence of differences in the ability of dopamine and norepinephrine to activate different human D4R variants. The potency of norepinephrine for adrenergic α2A receptor was only about 20-fold higher compared with D3R and D4R across the three functional assays.
U.S. Government work not protected by U.S. copyright.
Figures
References
-
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. (2010) Understanding wiring and volume transmission. Brain Res Brain Res Rev 64:137–159. - PubMed
-
- Antoni FA. (2012) New paradigms in cAMP signalling. Mol Cell Endocrinol 353:3–9. - PubMed
-
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HHM. (1995) Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem 65:1157–1165. - PubMed
-
- Beaulieu J-M, Gainetdinov RR. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

