Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival
- PMID: 26843191
- PMCID: PMC4870001
- DOI: 10.1126/scitranslmed.aad3305
Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival
Abstract
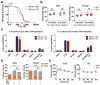
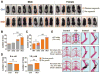
The absence of Bmal1, a core clock gene, results in a loss of circadian rhythms, an acceleration of aging, and a shortened life span in mice. To address the importance of circadian rhythms in the aging process, we generated conditional Bmal1 knockout mice that lacked the BMAL1 protein during adult life and found that wild-type circadian variations in wheel-running activity, heart rate, and blood pressure were abolished. Ocular abnormalities and brain astrogliosis were conserved irrespective of the timing of Bmal1 deletion. However, life span, fertility, body weight, blood glucose levels, and age-dependent arthropathy, which are altered in standard Bmal1 knockout mice, remained unaltered, whereas atherosclerosis and hair growth improved, in the conditional adult-life Bmal1 knockout mice, despite abolition of clock function. Hepatic RNA-Seq revealed that expression of oscillatory genes was dampened in the adult-life Bmal1 knockout mice, whereas overall gene expression was largely unchanged. Thus, many phenotypes in conventional Bmal1 knockout mice, hitherto attributed to disruption of circadian rhythms, reflect the loss of properties of BMAL1 that are independent of its role in the clock. These findings prompt reevaluation of the systemic consequences of disruption of the molecular clock.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
Figures

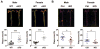


Similar articles
-
Generation of Myeloid-Specific Bmal1 Knockout Mice and Identification of Bmal1-Regulated Ferroptosis in Macrophages.Genesis. 2025 Apr;63(2):e70014. doi: 10.1002/dvg.70014. Genesis. 2025. PMID: 40197722
-
Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms.FASEB J. 2016 Apr;30(4):1634-42. doi: 10.1096/fj.15-282475. Epub 2015 Dec 23. FASEB J. 2016. PMID: 26700733 Free PMC article.
-
Voluntary Wheel Running Exercise Does Not Attenuate Circadian and Cardiac Dysfunction Caused by Conditional Deletion of Bmal1.J Biol Rhythms. 2023 Jun;38(3):290-304. doi: 10.1177/07487304231152398. Epub 2023 Feb 20. J Biol Rhythms. 2023. PMID: 36802963 Free PMC article.
-
The functional significance of the skeletal muscle clock: lessons from Bmal1 knockout models.Skelet Muscle. 2016 Oct 13;6:33. doi: 10.1186/s13395-016-0107-5. eCollection 2016. Skelet Muscle. 2016. PMID: 27752300 Free PMC article. Review.
-
Effects of BMAL1 Manipulation on the Brain's Master Circadian Clock and Behavior.Yale J Biol Med. 2019 Jun 27;92(2):251-258. eCollection 2019 Jun. Yale J Biol Med. 2019. PMID: 31249486 Free PMC article. Review.
Cited by
-
Sex Inclusion in Transcriptome Studies of Daily Rhythms.J Biol Rhythms. 2023 Feb;38(1):3-14. doi: 10.1177/07487304221134160. Epub 2022 Nov 23. J Biol Rhythms. 2023. PMID: 36419398 Free PMC article.
-
Survival is reduced when endogenous period deviates from 24 h in a non-human primate, supporting the circadian resonance theory.Sci Rep. 2020 Oct 22;10(1):18002. doi: 10.1038/s41598-020-75068-8. Sci Rep. 2020. PMID: 33093578 Free PMC article.
-
Bmal1 deletion in mice facilitates adaptation to disrupted light/dark conditions.JCI Insight. 2019 Apr 11;5(10):e125133. doi: 10.1172/jci.insight.125133. JCI Insight. 2019. PMID: 30973828 Free PMC article.
-
The wrinkling of time: Aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration.Neurobiol Dis. 2020 Jun;139:104832. doi: 10.1016/j.nbd.2020.104832. Epub 2020 Mar 13. Neurobiol Dis. 2020. PMID: 32179175 Free PMC article. Review.
-
Circadian control of ConA-induced acute liver injury and inflammatory response via Bmal1 regulation of Junb.JHEP Rep. 2023 Jul 22;5(11):100856. doi: 10.1016/j.jhepr.2023.100856. eCollection 2023 Nov. JHEP Rep. 2023. PMID: 37791375 Free PMC article.
References
-
- Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annu Rev Cell Dev Biol. 2001;17:215–253. - PubMed
-
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. - PubMed
-
- Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41:122–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

