Thermoreversible (Ionic-Liquid-Based) Aqueous Biphasic Systems
- PMID: 26843320
- PMCID: PMC4740753
- DOI: 10.1038/srep20276
Thermoreversible (Ionic-Liquid-Based) Aqueous Biphasic Systems
Abstract
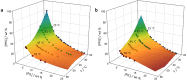
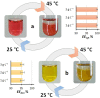
The ability to induce reversible phase transitions between homogeneous solutions and biphasic liquid-liquid systems, at pre-defined and suitable operating temperatures, is of crucial relevance in the design of separation processes. Ionic-liquid-based aqueous biphasic systems (IL-based ABS) have demonstrated superior performance as alternative extraction platforms, and their thermoreversible behaviour is here disclosed by the use of protic ILs. The applicability of the temperature-induced phase switching is further demonstrated with the complete extraction of two value-added proteins, achieved in a single-step. It is shown that these temperature-induced mono(bi)phasic systems are significantly more versatile than classical liquid-liquid systems which are constrained by their critical temperatures. IL-based ABS allow to work in a wide range of temperatures and compositions which can be tailored to fit the requirements of a given separation process.
Figures

References
-
- Bergbreiter D. E., Osburn P. L., Wilson A. & Sink E. M. Palladium-catalyzed C−C coupling under thermomorphic conditions. J. Am. Chem. Soc. 122, 9058–9064 (2000).
-
- Behr A., Henze G. & Schomäcker R. Thermoregulated liquid/liquid catalyst separation and recycling. Adv. Synth. Catal. 348, 1485–1495 (2006).
-
- Kohno Y. & Ohno H. Ionic liquid/water mixtures: from hostility to conciliation. Chem. Commun. 48, 7119–7130 (2012). - PubMed
-
- Jessop P. G., Mercer S. M. & Heldebrant D. J. CO2-triggered switchable solvents, surfactants, and other materials. Energy Environ. Sci. 5, 7240–7253 (2012).
-
- Luo J., Xin T. & Wang Y. A PEG bridged tertiary amine functionalized ionic liquid exhibiting thermoregulated reversible biphasic behavior with cyclohexane/isopropanol: synthesis and application in Knoevenagel condensation. New J. Chem. 37, 269–273 (2013).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

