Nanotechnology applications in thoracic surgery
- PMID: 26843431
- PMCID: PMC4913874
- DOI: 10.1093/ejcts/ezw002
Nanotechnology applications in thoracic surgery
Abstract
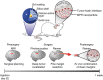
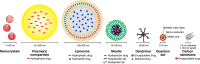
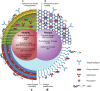
Nanotechnology is an emerging, rapidly evolving field with the potential to significantly impact care across the full spectrum of cancer therapy. Of note, several recent nanotechnological advances show particular promise to improve outcomes for thoracic surgical patients. A variety of nanotechnologies are described that offer possible solutions to existing challenges encountered in the detection, diagnosis and treatment of lung cancer. Nanotechnology-based imaging platforms have the ability to improve the surgical care of patients with thoracic malignancies through technological advances in intraoperative tumour localization, lymph node mapping and accuracy of tumour resection. Moreover, nanotechnology is poised to revolutionize adjuvant lung cancer therapy. Common chemotherapeutic drugs, such as paclitaxel, docetaxel and doxorubicin, are being formulated using various nanotechnologies to improve drug delivery, whereas nanoparticle (NP)-based imaging technologies can monitor the tumour microenvironment and facilitate molecularly targeted lung cancer therapy. Although early nanotechnology-based delivery systems show promise, the next frontier in lung cancer therapy is the development of 'theranostic' multifunctional NPs capable of integrating diagnosis, drug monitoring, tumour targeting and controlled drug release into various unifying platforms. This article provides an overview of key existing and emerging nanotechnology platforms that may find clinical application in thoracic surgery in the near future.
Keywords: Drug delivery; Imaging; Lung cancer; Nanoparticles; Nanotechnology; Theranostics.
© The Author 2016. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery. All rights reserved.
Figures



Similar articles
-
Recent progress in nanotechnology for cancer therapy.Chin J Cancer. 2010 Sep;29(9):775-80. doi: 10.5732/cjc.010.10075. Chin J Cancer. 2010. PMID: 20800018 Review.
-
Unique roles of nanotechnology in medicine and cancer-II.Indian J Cancer. 2015 Jan-Mar;52(1):1-9. doi: 10.4103/0019-509X.175591. Indian J Cancer. 2015. PMID: 26837958 Review.
-
Cancer nanotechnology: application of nanotechnology in cancer therapy.Drug Discov Today. 2010 Oct;15(19-20):842-50. doi: 10.1016/j.drudis.2010.08.006. Epub 2010 Aug 18. Drug Discov Today. 2010. PMID: 20727417 Review.
-
Applications of nanosystems to anticancer drug therapy (Part II. Dendrimers, micelles, lipid-based nanosystems).Recent Pat Anticancer Drug Discov. 2014 Jan;9(1):99-128. doi: 10.2174/1574891x113089990038. Recent Pat Anticancer Drug Discov. 2014. PMID: 23578193 Review.
-
From Diagnosis to Treatment: Clinical Applications of Nanotechnology in Thoracic Surgery.Thorac Surg Clin. 2016 May;26(2):215-28. doi: 10.1016/j.thorsurg.2015.12.009. Thorac Surg Clin. 2016. PMID: 27112260 Free PMC article. Review.
Cited by
-
Effects of different metastasis patterns, surgery and other factors on the prognosis of patients with stage IV non-small cell lung cancer: A Surveillance, Epidemiology, and End Results (SEER) linked database analysis.Oncol Lett. 2019 Jul;18(1):581-592. doi: 10.3892/ol.2019.10373. Epub 2019 May 20. Oncol Lett. 2019. PMID: 31289530 Free PMC article.
-
Design and Modular Construction of a Polymeric Nanoparticle for Targeted Atherosclerosis Positron Emission Tomography Imaging: A Story of 25% (64)Cu-CANF-Comb.Pharm Res. 2016 Oct;33(10):2400-10. doi: 10.1007/s11095-016-1963-8. Epub 2016 Jun 10. Pharm Res. 2016. PMID: 27286872 Free PMC article.
-
Engineered Nanomaterials for Improving the Nutritional Quality of Agricultural Products: A Review.Nanomaterials (Basel). 2022 Nov 27;12(23):4219. doi: 10.3390/nano12234219. Nanomaterials (Basel). 2022. PMID: 36500842 Free PMC article. Review.
-
Moving Beyond the Pillars of Cancer Treatment: Perspectives From Nanotechnology.Front Chem. 2020 Nov 10;8:598100. doi: 10.3389/fchem.2020.598100. eCollection 2020. Front Chem. 2020. PMID: 33240859 Free PMC article. Review.
-
Multimodal imaging of the receptor for advanced glycation end-products with molecularly targeted nanoparticles.Theranostics. 2018 Oct 5;8(18):5012-5024. doi: 10.7150/thno.24791. eCollection 2018. Theranostics. 2018. PMID: 30429883 Free PMC article.
References
-
- Alivisatos P. The use of nanocrystals in biological detection. Nat Biotechnol 2004;22:47–52. - PubMed
-
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 2005;5:161–71. - PubMed
-
- Cheng R, Meng F, Deng C, Klok HA, Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013;34:3647–57. - PubMed
-
- Colson YL, Grinstaff MW. Biologically responsive polymeric nanoparticles for drug delivery. Adv Mater 2012;24:3878–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

