GABAergic interneurons form transient layer-specific circuits in early postnatal neocortex
- PMID: 26843463
- PMCID: PMC4743032
- DOI: 10.1038/ncomms10584
GABAergic interneurons form transient layer-specific circuits in early postnatal neocortex
Abstract
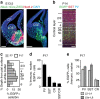
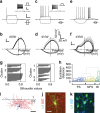
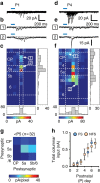
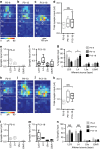
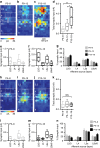
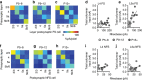
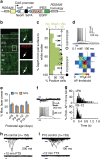
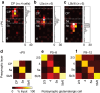
GABAergic interneurons play key roles in cortical circuits, yet little is known about their early connectivity. Here we use glutamate uncaging and a novel optogenetic strategy to track changes in the afferent and efferent synaptic connections of developing neocortical interneuron subtypes. We find that Nkx2-1-derived interneurons possess functional synaptic connections before emerging pyramidal cell networks. Subsequent interneuron circuit maturation is both subtype and layer dependent. Glutamatergic input onto fast spiking (FS), but not somatostatin-positive, non-FS interneurons increases over development. Interneurons of both subtype located in layers (L) 4 and 5b engage in transient circuits that disappear after the somatosensory critical period. These include a pathway mediated by L5b somatostatin-positive interneurons that specifically targets L4 during the first postnatal week. The innervation patterns of immature cortical interneuron circuits are thus neither static nor progressively strengthened but follow a layer-specific choreography of transient connections that differ from those of the adult brain.
Figures

References
-
- Ben-Ari Y., Khalilov I., Represa A. & Gozlan H. Interneurons set the tune of developing networks. Trends Neurosci. 27, 422–427 (2004). - PubMed
-
- Hensch T. K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888 (2005). - PubMed
-
- Markram H. et al.. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

