Time to Colonoscopy after Positive Fecal Blood Test in Four U.S. Health Care Systems
- PMID: 26843520
- PMCID: PMC4767632
- DOI: 10.1158/1055-9965.EPI-15-0470
Time to Colonoscopy after Positive Fecal Blood Test in Four U.S. Health Care Systems
Abstract
Background: To reduce colorectal cancer mortality, positive fecal blood tests must be followed by colonoscopy.
Methods: We identified 62,384 individuals ages 50 to 89 years with a positive fecal blood test between January 1, 2011 and December 31, 2012 in four health care systems within the Population-Based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium. We estimated the probability of follow-up colonoscopy and 95% confidence intervals (CI) using the Kaplan-Meier method. Overall differences in cumulative incidence of follow-up across health care systems were assessed with the log-rank test. HRs and 95% CIs were estimated from multivariate Cox proportional hazards models.
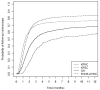
Results: Most patients who received a colonoscopy did so within 6 months of their positive fecal blood test, although follow-up rates varied across health care systems (P <0.001). Median days to colonoscopy ranged from 41 (95% CI, 40-41) to 174 (95% CI, 123-343); percent followed-up by 12 months ranged from 58.1% (95% CI, 51.6%-63.7%) to 83.8% (95% CI, 83.4%-84.3%) and differences across health care systems were also observed at 1, 2, 3, and 6 months. Increasing age and comorbidity score were associated with lower follow-up rates.
Conclusion: Individual characteristics and health care system were associated with colonoscopy after positive fecal blood tests. Patterns were consistent across health care systems, but proportions of patients receiving follow-up varied. These findings suggest that there is room to improve follow-up of positive colorectal cancer screening tests.
Impact: Understanding the timing of colonoscopy after positive fecal blood tests and characteristics associated with lack of follow-up may inform future efforts to improve follow-up.
©2016 American Association for Cancer Research.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- U S. Preventive Services Task Force. Screening for colorectal cancer: U.S Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. - PubMed
-
- Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. - PubMed
-
- Choi KS, Lee HY, Jun JK, Shin A, Park EC. Adherence to follow-up after a positive fecal occult blood test in an organized colorectal cancer screening program in Korea, 2004–2008. J Gastroenterol Hepatol. 2012;27:1070–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

