Cutaneous adverse events in multiple sclerosis patients treated with daclizumab
- PMID: 26843560
- PMCID: PMC4793779
- DOI: 10.1212/WNL.0000000000002417
Cutaneous adverse events in multiple sclerosis patients treated with daclizumab
Abstract
Objective: To analyze the spectrum and mechanisms of cutaneous adverse events (AEs) in patients with multiple sclerosis treated with daclizumab high-yield process (DAC-HYP).
Methods: A total of 31 participants in an institutional review board-approved open-label phase I study of DAC-HYP (NCT01143441) were prospectively evaluated over 42 months for development of cutaneous AEs. Participants provided written informed consent. Fifteen participants were naive to anti-CD25 therapy (cohort B), while 16 had received daclizumab (Zenapax; Hoffmann-La Roche) IV for 4-9 years (mean 5.8 years) prior to enrollment (cohort A). Immunohistochemistry was performed on pretreatment and posttreatment skin biopsies of normal-appearing skin (cohort B only) and on lesional biopsies in participants presenting with rash (both cohorts).
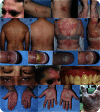
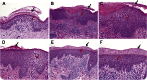
Results: Cutaneous AEs occurred in 77% of patients, the majority presenting with patches of eczema requiring no treatment. Moderate to severe rash developed in 6 participants (19%) and required discontinuation of DAC-HYP in 4 (13%). More severe rashes presented psoriasiform phenotype, but lesional biopsies lacked features of either psoriasis or drug hypersensitivity eruptions. Instead, irrespective of clinical severity, lesional biopsies showed nonspecific features of eczematous dermatitis, but with prominent CD56+ lymphocytic infiltrates. Pretreatment and posttreatment biopsies of normal-appearing skin demonstrated no histopathologic changes.
Conclusions: Observed cutaneous AEs are likely related to the immunomodulatory effects DAC-HYP exerts on innate lymphoid cells, including natural killer cells. Vigilance and timely management of skin reactions may prevent treatment discontinuation in participants with severe rash.
© 2016 American Academy of Neurology.
Figures



Similar articles
-
Safety and efficacy of daclizumab in relapsing-remitting multiple sclerosis: 3-year results from the SELECTED open-label extension study.BMC Neurol. 2016 Jul 26;16:117. doi: 10.1186/s12883-016-0635-y. BMC Neurol. 2016. PMID: 27461166 Free PMC article. Clinical Trial.
-
Daclizumab HYP versus Interferon Beta-1a in Relapsing Multiple Sclerosis.N Engl J Med. 2015 Oct 8;373(15):1418-28. doi: 10.1056/NEJMoa1501481. N Engl J Med. 2015. PMID: 26444729 Clinical Trial.
-
Daclizumab and its use in multiple sclerosis treatment.Drugs Today (Barc). 2017 Jan;53(1):7-18. doi: 10.1358/dot.2017.53.1.2570979. Drugs Today (Barc). 2017. PMID: 28387383 Review.
-
Daclizumab-induced adverse events in multiple organ systems in multiple sclerosis.Neurology. 2014 Mar 18;82(11):984-8. doi: 10.1212/WNL.0000000000000222. Epub 2014 Feb 14. Neurology. 2014. PMID: 24532277 Free PMC article.
-
Daclizumab: Development, Clinical Trials, and Practical Aspects of Use in Multiple Sclerosis.Neurotherapeutics. 2017 Oct;14(4):842-858. doi: 10.1007/s13311-017-0553-8. Neurotherapeutics. 2017. PMID: 28707278 Free PMC article. Review.
Cited by
-
Daclizumab.Hosp Pharm. 2016 Dec;51(11):928-939. doi: 10.1310/hpj5111-928. Hosp Pharm. 2016. PMID: 28057953 Free PMC article.
-
A comprehensive review on the treatment approaches of multiple sclerosis: currently and in the future.Inflamm Res. 2019 Jan;68(1):25-38. doi: 10.1007/s00011-018-1185-0. Epub 2018 Sep 3. Inflamm Res. 2019. PMID: 30178100 Review.
-
Daclizumab high-yield process in the treatment of relapsing-remitting multiple sclerosis.Ther Adv Neurol Disord. 2017 Jan;10(1):67-75. doi: 10.1177/1756285616671887. Epub 2016 Oct 19. Ther Adv Neurol Disord. 2017. PMID: 28450896 Free PMC article. Review.
-
Monoclonal Antibodies as Neurological Therapeutics.Pharmaceuticals (Basel). 2021 Jan 26;14(2):92. doi: 10.3390/ph14020092. Pharmaceuticals (Basel). 2021. PMID: 33530460 Free PMC article. Review.
-
Daclizumab Therapy for Multiple Sclerosis.Cold Spring Harb Perspect Med. 2019 May 1;9(5):a034470. doi: 10.1101/cshperspect.a034470. Cold Spring Harb Perspect Med. 2019. PMID: 29661806 Free PMC article. Review.
References
-
- Wynn D, Kaufman M, Montalban X, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol 2010;9:381–390. - PubMed
-
- Gold R, Giovannoni G, Selmaj K, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet 2013;381:2167–2175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
