Inactivation of BRCA2 in human cancer cells identifies a subset of tumors with enhanced sensitivity towards death receptor-mediated apoptosis
- PMID: 26843614
- PMCID: PMC4891053
- DOI: 10.18632/oncotarget.7053
Inactivation of BRCA2 in human cancer cells identifies a subset of tumors with enhanced sensitivity towards death receptor-mediated apoptosis
Abstract
Purpose: DNA repair defects due to detrimental BRCA2-mutations confer increased susceptibility towards DNA interstrand-crosslinking (ICL) agents and define patient subpopulations for individualized genotype-based cancer therapy. However, due to the side effects of these drugs, there is a need to identify additional agents, which could be used alone or in combination with ICL-agents. Therefore, we investigated whether BRCA2-mutations might also increase the sensitivity towards TRAIL-receptors (TRAIL-R)-targeting compounds.
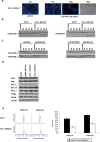
Experimental design: Two independent model systems were applied: a BRCA2 gene knockout and a BRCA2 gene complementation model. The effects of TRAIL-R-targeting compounds and ICL-agents on cell viability, apoptosis and cell cycle distribution were compared in BRCA2-proficient versus-deficient cancer cells in vitro. In addition, the effects of the TRAIL-R2-targeting antibody LBY135 were assessed in vivo using a murine tumor xenograft model.
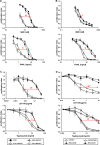
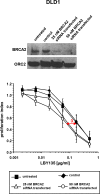
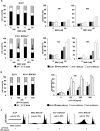
Results: BRCA2-deficient cancer cells displayed an increased sensitivity towards TRAIL-R-targeting agents. These effects exceeded and were mechanistically distinguishable from the well-established effects of ICL-agents. In vitro, ICL-agents expectedly induced an early cell cycle arrest followed by delayed apoptosis, whereas TRAIL-R-targeting compounds caused early apoptosis without prior cell cycle arrest. In vivo, treatment with LBY135 significantly reduced the tumor growth of BRCA2-deficient cancer cells in a xenograft model.
Conclusions: BRCA2 mutations strongly increase the in vitro- and in vivo-sensitivity of cancer cells towards TRAIL-R-mediated apoptosis. This effect is mechanistically distinguishable from the well-established ICL-hypersensitivity of BRCA2-deficient cells. Our study thus defines a new genetic subpopulation of cancers susceptible towards TRAIL-R-targeting compounds, which could facilitate novel therapeutic approaches for patients with BRCA2-deficient tumors.
Keywords: BRCA2; TRAIL; apoptosis; gene targeting; targeted therapy.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

