[68Ga]Pentixafor-PET/CT for imaging of chemokine receptor 4 expression in small cell lung cancer--initial experience
- PMID: 26843617
- PMCID: PMC4891040
- DOI: 10.18632/oncotarget.7063
[68Ga]Pentixafor-PET/CT for imaging of chemokine receptor 4 expression in small cell lung cancer--initial experience
Abstract
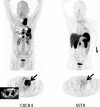
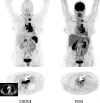
Chemokine receptor CXCR4 is a key factor for tumor growth and metastasis in several types of human cancer. This study investigated the feasibility of CXCR4-directed imaging of small cell lung cancer (SCLC) with positron emission tomography/computed tomography (PET/CT) using the radiolabelled chemokine ligand [68Ga]Pentixafor. 10 patients with primarily diagnosed (n=3) or pre-treated (n=7) SCLC (n=9) or large cell neuroendocrine carcinoma of the lung (LCNEC, n=1) underwent [68Ga]Pentixafor-PET/CT. 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG, n=6) and/or somatostatin receptor (SSTR)-directed PET/CT with [68Ga]DOTATOC (n=5) and immunohistochemistry (n=10) served as standards of reference. CXCR4-PET was positive in 8/10 patients and revealed more lesions with significantly higher tumor-to-background ratios than SSTR-PET. Two patients who were positive on [18F]FDG-PET were missed by CXCR4-PET, in the remainder [68Ga]Pentixafor detected an equal (n=2) or higher (n=2) number of lesions. CXCR4 expression of tumor lesions could be confirmed by immunohistochemistry. Non-invasive imaging of CXCR4 expression in SCLC is feasible. [68Ga]Pentixafor as a novel PET tracer might serve as readout for confirmation of CXCR4 expression as prerequisite for potential CXCR4-directed treatment including receptor-radio(drug)peptide therapy.
Keywords: CXCR4; PET; SCLC; molecular imaging; small cell lung cancer.
Conflict of interest statement
HJW is the founder and shareholder of Scintomics. SK is CEO of Scintomics. All other authors declare no conflicts of interest.
Figures

References
-
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. Journal of clinical oncology. 2006;24:4539–4544. - PubMed
-
- Cuffe S, Moua T, Summerfield R, Roberts H, Jett J, Shepherd FA. Characteristics and outcomes of small cell lung cancer patients diagnosed during two lung cancer computed tomographic screening programs in heavy smokers. Journal of thoracic oncology. 2011;6:818–822. - PubMed
-
- Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, Werner-Wasik M, Videtic GM, Garces YI, Choy H. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. International journal of radiation oncology, biology, physics. 2011;81:77–84. - PMC - PubMed
-
- Foster NR, Qi Y, Shi Q, Krook JE, Kugler JW, Jett JR, Molina JR, Schild SE, Adjei AA, Mandrekar SJ. Tumor response and progression-free survival as potential surrogate endpoints for overall survival in extensive stage small-cell lung cancer: findings on the basis of North Central Cancer Treatment Group trials. Cancer. 2011;117:1262–1271. - PMC - PubMed
-
- Fruh M, De Ruysscher D, Popat S, Crino L, Peters S, Felip E, Group EGW. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology. 2013;24(Suppl 6):vi99–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

