Evolving roles of circadian rhythms in liver homeostasis and pathology
- PMID: 26843619
- PMCID: PMC4890992
- DOI: 10.18632/oncotarget.7065
Evolving roles of circadian rhythms in liver homeostasis and pathology
Abstract
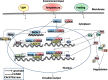
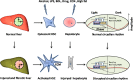
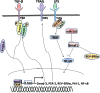
Circadian clock in mammals is determined by a core oscillator in the suprachiasmatic nucleus (SCN) of the hypothalamus and synchronized peripheral clocks in other tissues. The coherent timing systems could sustain robust output of circadian rhythms in response to the entrainment controlled environmentally. Disparate approaches have discovered that clock genes and clock-controlled genes (CCGs) exist in nearly all mammalian cell types and are essential for establishing the mechanisms and complexity of internal time-keeping systems. Accumulating evidence demonstrates that the control of homeostasis and pathology in the liver involves intricate loops of transcriptional and post-translational regulation of clock genes expression. This review will focus on the recent advances with great importance concerning clock rhythms linking liver homeostasis and diseases. We particularly highlight what is currently known of the evolving insights into the mechanisms underlying circadian clock . Eventually , findings during recent years in the field might prompt new circadian-related chronotherapeutic strategies for the diagnosis and treatment of liver diseases by coupling these processes.
Keywords: circadian rhythms; epigenetic modifications; hepatocellular carcinoma; liver fibrosis; liver metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Circadian rhythms of liver physiology and disease: experimental and clinical evidence.Nat Rev Gastroenterol Hepatol. 2016 Apr;13(4):217-26. doi: 10.1038/nrgastro.2016.8. Epub 2016 Feb 24. Nat Rev Gastroenterol Hepatol. 2016. PMID: 26907879 Review.
-
Circadian desynchrony and glucose metabolism.J Pineal Res. 2024 May;76(4):e12956. doi: 10.1111/jpi.12956. J Pineal Res. 2024. PMID: 38695262 Review.
-
Sleep timing and the circadian clock in mammals: Past, present and the road ahead.Semin Cell Dev Biol. 2022 Jun;126:3-14. doi: 10.1016/j.semcdb.2021.05.034. Epub 2021 Jun 4. Semin Cell Dev Biol. 2022. PMID: 34092510 Free PMC article. Review.
-
Entrainment of circadian clocks in mammals by arousal and food.Essays Biochem. 2011 Jun 30;49(1):119-36. doi: 10.1042/bse0490119. Essays Biochem. 2011. PMID: 21819388
-
The suprachiasmatic nucleus and the circadian time-keeping system revisited.Brain Res Brain Res Rev. 2000 Aug;33(1):34-77. doi: 10.1016/s0165-0173(00)00025-4. Brain Res Brain Res Rev. 2000. PMID: 10967353 Review.
Cited by
-
Relationship between shift work and liver enzymes: a cross-sectional study based on the Korea National Health and Examination Survey (2007-2015).Ann Occup Environ Med. 2019 Jul 31;31:e15. doi: 10.35371/aoem.2019.31.e15. eCollection 2019. Ann Occup Environ Med. 2019. PMID: 31583106 Free PMC article.
-
Chrononutrition Applied to Diabetes Management: A Paradigm Shift Long Delayed.Diabetes Spectr. 2018 Nov;31(4):349-353. doi: 10.2337/ds18-0014. Diabetes Spectr. 2018. PMID: 30510391 Free PMC article. No abstract available.
-
Development of a circadian-related prognostic signature highlights RBM17 as a stemness regulator in liver cancer.Cancer Cell Int. 2025 Jun 13;25(1):211. doi: 10.1186/s12935-025-03843-6. Cancer Cell Int. 2025. PMID: 40514677 Free PMC article.
-
Melatonin attenuates smoking-induced hyperglycemia via preserving insulin secretion and hepatic glycogen synthesis in rats.J Pineal Res. 2018 May;64(4):e12475. doi: 10.1111/jpi.12475. Epub 2018 Mar 25. J Pineal Res. 2018. PMID: 29437243 Free PMC article.
-
Chronomedicine and type 2 diabetes: shining some light on melatonin.Diabetologia. 2017 May;60(5):808-822. doi: 10.1007/s00125-016-4175-1. Epub 2016 Dec 16. Diabetologia. 2017. PMID: 27981356 Review.
References
-
- Vanin S, Bhutani S, Montelli S, Menegazzi P, Green EW, Pegoraro M, Sandrelli F, Costa R, Kyriacou CP. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature. 2012;484:371–375. - PubMed
-
- Whalley K. Circadian rhythms: Temperature training. Nat Rev Neurosci. 2013;14:380–380.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

