An In Vivo Pharmacological Screen Identifies Cholinergic Signaling as a Therapeutic Target in Glial-Based Nervous System Disease
- PMID: 26843629
- PMCID: PMC4737762
- DOI: 10.1523/JNEUROSCI.0256-15.2016
An In Vivo Pharmacological Screen Identifies Cholinergic Signaling as a Therapeutic Target in Glial-Based Nervous System Disease
Abstract
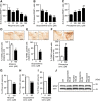
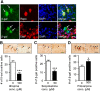
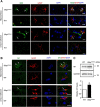
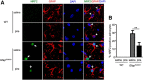
The role that glia play in neurological disease is poorly understood but increasingly acknowledged to be critical in a diverse group of disorders. Here we use a simple genetic model of Alexander disease, a progressive and severe human degenerative nervous system disease caused by a primary astroglial abnormality, to perform an in vivo screen of 1987 compounds, including many FDA-approved drugs and natural products. We identify four compounds capable of dose-dependent inhibition of nervous system toxicity. Focusing on one of these hits, glycopyrrolate, we confirm the role for muscarinic cholinergic signaling in pathogenesis using additional pharmacologic reagents and genetic approaches. We further demonstrate that muscarinic cholinergic signaling works through downstream Gαq to control oxidative stress and death of neurons and glia. Importantly, we document increased muscarinic cholinergic receptor expression in Alexander disease model mice and in postmortem brain tissue from Alexander disease patients, and that blocking muscarinic receptors in Alexander disease model mice reduces oxidative stress, emphasizing the translational significance of our findings. We have therefore identified glial muscarinic signaling as a potential therapeutic target in Alexander disease, and possibly in other gliopathic disorders as well.
Significance statement: Despite the urgent need for better treatments for neurological diseases, drug development for these devastating disorders has been challenging. The effectiveness of traditional large-scale in vitro screens may be limited by the lack of the appropriate molecular, cellular, and structural environment. Using a simple Drosophila model of Alexander disease, we performed a moderate throughput chemical screen of FDA-approved drugs and natural compounds, and found that reducing muscarinic cholinergic signaling ameliorated clinical symptoms and oxidative stress in Alexander disease model flies and mice. Our work demonstrates that small animal models are valuable screening tools for therapeutic compound identification in complex human diseases and that existing drugs can be a valuable resource for drug discovery given their known pharmacological and safety profiles.
Keywords: Alexander disease; Drosophila; chemical screen; cholinergic signaling; glia.
Copyright © 2016 the authors 0270-6474/16/361445-11$15.00/0.
Figures




Similar articles
-
Nitric oxide mediates glial-induced neurodegeneration in Alexander disease.Nat Commun. 2015 Nov 26;6:8966. doi: 10.1038/ncomms9966. Nat Commun. 2015. PMID: 26608817 Free PMC article.
-
Protein misfolding and oxidative stress promote glial-mediated neurodegeneration in an Alexander disease model.J Neurosci. 2011 Feb 23;31(8):2868-77. doi: 10.1523/JNEUROSCI.3410-10.2011. J Neurosci. 2011. PMID: 21414908 Free PMC article.
-
Tissue and cellular rigidity and mechanosensitive signaling activation in Alexander disease.Nat Commun. 2018 May 15;9(1):1899. doi: 10.1038/s41467-018-04269-7. Nat Commun. 2018. PMID: 29765022 Free PMC article.
-
Alzheimer's disease: Targeting the Cholinergic System.Curr Neuropharmacol. 2016;14(1):101-15. doi: 10.2174/1570159x13666150716165726. Curr Neuropharmacol. 2016. PMID: 26813123 Free PMC article. Review.
-
[Mechanism of Microglial Surveillance and Protection against Alexander Disease Pathology].Brain Nerve. 2025 Mar;77(3):281-288. doi: 10.11477/mf.188160960770030281. Brain Nerve. 2025. PMID: 40064494 Review. Japanese.
Cited by
-
Anastasis Drives Senescence and Non-Cell Autonomous Neurodegeneration in the Astrogliopathy Alexander Disease.J Neurosci. 2022 Mar 23;42(12):2584-2597. doi: 10.1523/JNEUROSCI.1659-21.2021. Epub 2022 Feb 1. J Neurosci. 2022. PMID: 35105675 Free PMC article.
-
Drosophila Glia: Models for Human Neurodevelopmental and Neurodegenerative Disorders.Int J Mol Sci. 2020 Jul 9;21(14):4859. doi: 10.3390/ijms21144859. Int J Mol Sci. 2020. PMID: 32660023 Free PMC article. Review.
-
Lrrk promotes tau neurotoxicity through dysregulation of actin and mitochondrial dynamics.PLoS Biol. 2018 Dec 20;16(12):e2006265. doi: 10.1371/journal.pbio.2006265. eCollection 2018 Dec. PLoS Biol. 2018. PMID: 30571694 Free PMC article.
-
Nutrigenomics as a tool to study the impact of diet on aging and age-related diseases: the Drosophila approach.Genes Nutr. 2019 May 2;14:12. doi: 10.1186/s12263-019-0638-6. eCollection 2019. Genes Nutr. 2019. PMID: 31073342 Free PMC article. Review.
-
Glial contributions to neuronal health and disease: new insights from Drosophila.Curr Opin Neurobiol. 2017 Dec;47:162-167. doi: 10.1016/j.conb.2017.10.008. Epub 2017 Nov 6. Curr Opin Neurobiol. 2017. PMID: 29096245 Free PMC article. Review.
References
-
- Anisuzzaman AS, Uwada J, Masuoka T, Yoshiki H, Nishio M, Ikegaya Y, Takahashi N, Matsuki N, Fujibayashi Y, Yonekura Y, Momiyama T, Muramatsu I. Novel contribution of cell surface and intracellular M1-muscarinic acetylcholine receptors to synaptic plasticity in hippocampus. J Neurochem. 2013;126:360–371. doi: 10.1111/jnc.12306. - DOI - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
