Blockade of Astrocytic Calcineurin/NFAT Signaling Helps to Normalize Hippocampal Synaptic Function and Plasticity in a Rat Model of Traumatic Brain Injury
- PMID: 26843634
- PMCID: PMC4737766
- DOI: 10.1523/JNEUROSCI.1930-15.2016
Blockade of Astrocytic Calcineurin/NFAT Signaling Helps to Normalize Hippocampal Synaptic Function and Plasticity in a Rat Model of Traumatic Brain Injury
Abstract
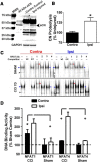
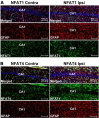
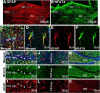
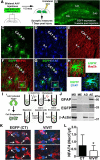
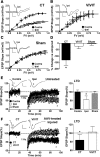
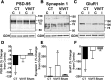
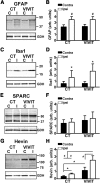
Increasing evidence suggests that the calcineurin (CN)-dependent transcription factor NFAT (Nuclear Factor of Activated T cells) mediates deleterious effects of astrocytes in progressive neurodegenerative conditions. However, the impact of astrocytic CN/NFAT signaling on neural function/recovery after acute injury has not been investigated extensively. Using a controlled cortical impact (CCI) procedure in rats, we show that traumatic brain injury is associated with an increase in the activities of NFATs 1 and 4 in the hippocampus at 7 d after injury. NFAT4, but not NFAT1, exhibited extensive labeling in astrocytes and was found throughout the axon/dendrite layers of CA1 and the dentate gyrus. Blockade of the astrocytic CN/NFAT pathway in rats using adeno-associated virus (AAV) vectors expressing the astrocyte-specific promoter Gfa2 and the NFAT-inhibitory peptide VIVIT prevented the injury-related loss of basal CA1 synaptic strength and key synaptic proteins and reduced the susceptibility to induction of long-term depression. In conjunction with these seemingly beneficial effects, VIVIT treatment elicited a marked increase in the expression of the prosynaptogenic factor SPARCL1 (hevin), especially in hippocampal tissue ipsilateral to the CCI injury. However, in contrast to previous work on Alzheimer's mouse models, AAV-Gfa2-VIVIT had no effects on the levels of GFAP and Iba1, suggesting that synaptic benefits of VIVIT were not attributable to a reduction in glial activation per se. Together, the results implicate the astrocytic CN/NFAT4 pathway as a key mechanism for disrupting synaptic remodeling and homeostasis in the hippocampus after acute injury.
Significance statement: Similar to microglia, astrocytes become strongly "activated" with neural damage and exhibit numerous morphologic/biochemical changes, including an increase in the expression/activity of the protein phosphatase calcineurin. Using adeno-associated virus (AAV) to inhibit the calcineurin-dependent activation of the transcription factor NFAT (Nuclear Factor of Activated T cells) selectively, we have shown that activated astrocytes contribute to neural dysfunction in animal models characterized by progressive/chronic neuropathology. Here, we show that the suppression of astrocytic calcineurin/NFATs helps to protect synaptic function and plasticity in an animal model in which pathology arises from a single traumatic brain injury. The findings suggest that at least some astrocyte functions impair recovery after trauma and may provide druggable targets for treating victims of acute nervous system injury.
Keywords: astrocytes; brain injury; calcineurin; calcium; glia; synapse.
Copyright © 2016 the authors 0270-6474/16/361503-14$15.00/0.
Figures
Similar articles
-
Calcineurin/NFAT Signaling in Activated Astrocytes Drives Network Hyperexcitability in Aβ-Bearing Mice.J Neurosci. 2017 Jun 21;37(25):6132-6148. doi: 10.1523/JNEUROSCI.0877-17.2017. Epub 2017 May 30. J Neurosci. 2017. PMID: 28559377 Free PMC article.
-
Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity.J Biol Chem. 2008 Aug 8;283(32):21953-64. doi: 10.1074/jbc.M800148200. Epub 2008 Jun 9. J Biol Chem. 2008. PMID: 18541537 Free PMC article.
-
Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer's disease.J Neurosci. 2012 Nov 14;32(46):16129-40. doi: 10.1523/JNEUROSCI.2323-12.2012. J Neurosci. 2012. PMID: 23152597 Free PMC article.
-
Astrocyte Activation and the Calcineurin/NFAT Pathway in Cerebrovascular Disease.Front Aging Neurosci. 2018 Sep 21;10:287. doi: 10.3389/fnagi.2018.00287. eCollection 2018. Front Aging Neurosci. 2018. PMID: 30297999 Free PMC article. Review.
-
The calcineurin/NFAT signaling pathway: a novel therapeutic target in leukemia and solid tumors.Cell Cycle. 2008 Feb 1;7(3):297-303. doi: 10.4161/cc.7.3.5357. Epub 2007 Nov 23. Cell Cycle. 2008. PMID: 18235236 Review.
Cited by
-
Propofol maintains Th17/Treg cell balance and reduces inflammation in rats with traumatic brain injury via the miR‑145‑3p/NFATc2/NF‑κB axis.Int J Mol Med. 2021 Jul;48(1):135. doi: 10.3892/ijmm.2021.4968. Epub 2021 May 26. Int J Mol Med. 2021. PMID: 34036377 Free PMC article.
-
Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury.Cell Commun Signal. 2020 Apr 15;18(1):62. doi: 10.1186/s12964-020-00549-2. Cell Commun Signal. 2020. PMID: 32293472 Free PMC article. Review.
-
An update on the association between traumatic brain injury and Alzheimer's disease: Focus on Tau pathology and synaptic dysfunction.Neurosci Biobehav Rev. 2021 Jan;120:372-386. doi: 10.1016/j.neubiorev.2020.10.020. Epub 2020 Nov 7. Neurosci Biobehav Rev. 2021. PMID: 33171143 Free PMC article. Review.
-
The TNFα-Transgenic Rat: Hippocampal Synaptic Integrity, Cognition, Function, and Post-Ischemic Cell Loss.PLoS One. 2016 May 4;11(5):e0154721. doi: 10.1371/journal.pone.0154721. eCollection 2016. PLoS One. 2016. PMID: 27144978 Free PMC article.
-
Construction of rat spinal cord injury model based on Allen's animal model.Saudi J Biol Sci. 2019 Dec;26(8):2122-2126. doi: 10.1016/j.sjbs.2019.09.033. Epub 2019 Oct 16. Saudi J Biol Sci. 2019. PMID: 31889806 Free PMC article.
References
-
- Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H, 3rd, Kraner SD, Norris CM. Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
