Persistent Electrical Activity in Primary Nociceptors after Spinal Cord Injury Is Maintained by Scaffolded Adenylyl Cyclase and Protein Kinase A and Is Associated with Altered Adenylyl Cyclase Regulation
- PMID: 26843647
- PMCID: PMC4737775
- DOI: 10.1523/JNEUROSCI.0895-15.2016
Persistent Electrical Activity in Primary Nociceptors after Spinal Cord Injury Is Maintained by Scaffolded Adenylyl Cyclase and Protein Kinase A and Is Associated with Altered Adenylyl Cyclase Regulation
Abstract
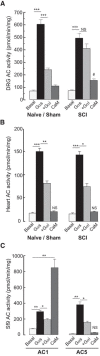
Little is known about intracellular signaling mechanisms that persistently excite neurons in pain pathways. Persistent spontaneous activity (SA) generated in the cell bodies of primary nociceptors within dorsal root ganglia (DRG) has been found to make major contributions to chronic pain in a rat model of spinal cord injury (SCI) (Bedi et al., 2010; Yang et al., 2014). The occurrence of SCI-induced SA in a large fraction of DRG neurons and the persistence of this SA long after dissociation of the neurons provide an opportunity to define intrinsic cell signaling mechanisms that chronically drive SA in pain pathways. The present study demonstrates that SCI-induced SA requires continuing activity of adenylyl cyclase (AC) and cAMP-dependent protein kinase (PKA), as well as a scaffolded complex containing AC5/6, A-kinase anchoring protein 150 (AKAP150), and PKA. SCI caused a small but significant increase in the expression of AKAP150 but not other AKAPs. DRG membranes isolated from SCI animals revealed a novel alteration in the regulation of AC. AC activity stimulated by Ca(2+)-calmodulin increased, while the inhibition of AC activity by Gαi showed an unexpected and dramatic decrease after SCI. Localized enhancement of the activity of AC within scaffolded complexes containing PKA is likely to contribute to chronic pathophysiological consequences of SCI, including pain, that are promoted by persistent hyperactivity in DRG neurons.
Significance statement: Chronic neuropathic pain is a major clinical problem with poorly understood mechanisms and inadequate treatments. Recent findings indicate that chronic pain in a rat SCI model depends upon hyperactivity in dorsal root ganglia (DRG) neurons. Although cAMP signaling is involved in many forms of neural plasticity, including hypersensitivity of nociceptors in the presence of inflammatory mediators, our finding that continuing cAMP-PKA signaling is required for persistent SA months after SCI and long after isolation of nociceptors is surprising. The dependence of ongoing SA upon AKAP150 and AC5/6 was unknown. The discovery of a dramatic decrease in Gαi inhibition of AC activity after SCI is novel for any physiological system and potentially has broad implications for understanding chronic pain mechanisms.
Keywords: A-kinase anchoring protein; DRG; cAMP; chronic pain; hyperexcitability; spontaneous activity.
Copyright © 2016 the authors 0270-6474/16/361660-09$15.00/0.
Figures



References
-
- Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci. 2010;30:14870–14882. doi: 10.1523/JNEUROSCI.2428-10.2010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
