Sex Differences in GABAA Signaling in the Periaqueductal Gray Induced by Persistent Inflammation
- PMID: 26843648
- PMCID: PMC4737776
- DOI: 10.1523/JNEUROSCI.1928-15.2016
Sex Differences in GABAA Signaling in the Periaqueductal Gray Induced by Persistent Inflammation
Abstract
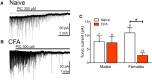
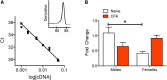
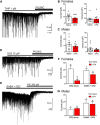
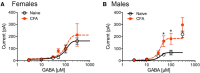
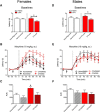
The ventrolateral periaqueductal gray (vlPAG) is a key structure in the descending pain modulatory circuit. Activation of the circuit occurs via disinhibition of GABAergic inputs onto vlPAG output neurons. In these studies, we tested the hypothesis that GABAergic inhibition is increased during persistent inflammation, dampening activation of the descending circuit from the vlPAG. Our results indicate that persistent inflammation induced by Complete Freund's adjuvant (CFA) modulates GABA signaling differently in male and female rats. CFA treatment results in increased presynaptic GABA release but decreased high-affinity tonic GABAA currents in female vlPAG neurons. These effects are not observed in males. The tonic currents in the vlPAG are dependent on GABA transporter activity and are modulated by agonists that activate GABAA receptors containing the δ subunit. The GABAA δ agonist THIP (gaboxadol) induced similar amplitude currents in naive and CFA-treated rats. In addition, a positive allosteric modulator of the GABAA δ subunit, DS2 (4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl]benzamide), increased tonic currents. These results indicate that GABAA δ receptors remain on the cell surface but are less active in CFA-treated female rats. In vivo behavior studies showed that morphine induced greater antinociception in CFA-treated females that was reversed with microinjections of DS2 directly into the vlPAG. DS2 did not affect morphine antinociception in naive or CFA-treated male rats. Together, these data indicate that sex-specific adaptations in GABAA receptor signaling modulate opioid analgesia in persistent inflammation. Antagonists of GABAA δ receptors may be a viable strategy for reducing pain associated with persistent inflammation, particularly in females.
Significance statement: These studies demonstrate that GABA signaling is modulated in the ventrolateral periaqueductal gray by persistent inflammation differently in female and male rats. Our results indicate that antagonists or negative allosteric modulators of GABAA δ receptors may be an effective strategy to alleviate chronic inflammatory pain and promote opioid antinociception, especially in females.
Keywords: GABAA; chronic pain; descending pain control; opioid; sex difference; tonic current.
Copyright © 2016 the authors 0270-6474/16/361669-13$15.00/0.
Figures



References
-
- Barakat L, Bordey A. GAT-1 and reversible GABA transport in Bergmann glia in slices. J Neurophysiol. 2002;88:1407–1419. - PubMed
-
- Barnard EA, Seeburg PH. Structural basis of the GABA-activated chloride channel: molecular biology and molecular electrophysiology. Adv Biochem Psychopharmacol. 1988;45:1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
