Swain-Scott Relationships for Nucleophile Addition to Ring-Substituted Phenonium Ions
- PMID: 26843657
- PMCID: PMC4734394
- DOI: 10.1139/cjc-2014-0337
Swain-Scott Relationships for Nucleophile Addition to Ring-Substituted Phenonium Ions
Abstract
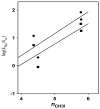
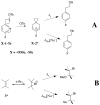
The products of the reactions of 2-(4-methoxyphenyl)ethyl tosylate (MeO-1-Ts) and 2-(4-methyphenyl)ethyl tosylate (Me-1-Ts) with nucleophilic anions were determined for reactions in 50/50 (v/v) trifluoroethanol/water at 25°C. In many cases the nucleophile selectivity kNu/ks (M-1) for reactions of nucleophile and solvent, calculated from the ratio of product yields, depends upon [Nu-]. This demonstrates the existence of competing reaction pathways, which show different selectivities for reactions with nucleophiles. A carbon-13 NMR analysis of the products of the reactions of substrate enriched with carbon-13 at the α-carbon, X-1-[α-13C]Ts, (X = -OCH3, -Me) with nucleophilic anions in 50/50 (v/v) trifluoroethanol/water at 25°C shows the formation of X-1-[β-13C]OH, X-1-[β-13C]OCH2CF3 and X-1-[β-13C]Nu (Nu = Br, Cl, CH3CO2, Cl2CHCO2) from the trapping of symmetrical 4-substituted phenonium ion reaction intermediates X-2+ . The observation of excess label in the α-position, [α-13C]/[β-13C] > 1.0, for both the water and nucleophile adducts, shows that water and anionic nucleophiles also react by direct substitution at X-1-[α-13C]Ts. The ratios of product yields, [α-13C]/[β-13C], and observed nucleophile selectivity (kNu/ks)obs were used to dissect the contribution of direct nucleophile addition at Me-1-Ts and trapping of X-2+ to the product yields. The product yields from partitioning of the intermediate gave the nucleophile selectivity kNu/ks (M-1) for X-2+ . Swain-Scott plots of log (kNu/ks) are correlated by slopes of s = 0.78 and s = 0.73 for reactions of MeO-2+ and Me-2+ , respectively. This shows that the sensitivity of bimolecular substitution at X-2+ to changes in nucleophile reactivity is smaller than for nucleophilic substitution at the methyl iodide. Evidence is presented that nucleophile addition to X-2+ proceeds through an "exploded" transition state.
Figures



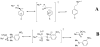
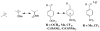
Similar articles
-
Formation and Mechanism for Reactions of Ring-Substituted Phenonium Ions in Aqueous Solution.J Phys Org Chem. 2016 Nov;29(11):557-564. doi: 10.1002/poc.3510. Epub 2015 Nov 30. J Phys Org Chem. 2016. PMID: 27812239 Free PMC article.
-
Substituent Effects on the Formation and Nucleophile Selectivity of Ring-Substituted Phenonium Ions in Aqueous Solution.J Phys Org Chem. 2013 Dec 1;26(12):970-976. doi: 10.1002/poc.3145. J Phys Org Chem. 2013. PMID: 24511183 Free PMC article.
-
Recent advances in our mechanistic understanding of S(N)V reactions.Acc Chem Res. 2009 Aug 18;42(8):993-1003. doi: 10.1021/ar900048q. Acc Chem Res. 2009. PMID: 19522460
-
Negative ion gas-phase chemistry of arenes.Mass Spectrom Rev. 2016 Jan-Feb;35(1):123-46. doi: 10.1002/mas.21467. Epub 2015 Apr 6. Mass Spectrom Rev. 2016. PMID: 25851641 Review.
-
Linear Free Energy Relationships for Enzymatic Reactions: Fresh Insight from a Venerable Probe.Acc Chem Res. 2021 May 18;54(10):2532-2542. doi: 10.1021/acs.accounts.1c00147. Epub 2021 May 3. Acc Chem Res. 2021. PMID: 33939414 Free PMC article. Review.
Cited by
-
Formation and Mechanism for Reactions of Ring-Substituted Phenonium Ions in Aqueous Solution.J Phys Org Chem. 2016 Nov;29(11):557-564. doi: 10.1002/poc.3510. Epub 2015 Nov 30. J Phys Org Chem. 2016. PMID: 27812239 Free PMC article.
References
-
- More O’Ferrall R. Adv Phys Org Chem. 2010;44:19.
-
- McClelland RA. Tetrahedron. 1996;52:6823.
-
- Richard JP. Tetrahedron. 1995;51:1535.
-
- Toteva MM, Richard JP. J Am Chem Soc. 1996;118:11434.
-
- Amyes TL, Jencks WP. J Am Chem Soc. 1989;111:7888.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
