Cell Line-Dependent Variability of Coordinate Expression of p75NTR and CRABP1 and Modulation of Effects of Fenretinide on Neuroblastoma Cells
- PMID: 26843908
- PMCID: PMC4710897
- DOI: 10.1155/2016/7568287
Cell Line-Dependent Variability of Coordinate Expression of p75NTR and CRABP1 and Modulation of Effects of Fenretinide on Neuroblastoma Cells
Abstract
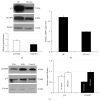
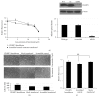
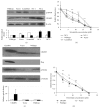
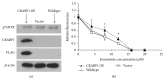
Neuroblastoma is a childhood neural crest tumor. Fenretinide, a retinoic acid analogue, induces accumulation of mitochondrial reactive oxygen species and consequent apoptosis in neuroblastoma cells. The p75 neurotrophin receptor (p75NTR) enhances the antineuroblastoma cell efficacy of fenretinide in vitro. We examined the role of the retinoid binding protein, CRABP1, in p75NTR-mediated potentiation of the efficacy of fenretinide. Knockdown and overexpression, respectively, of either p75NTR or CRABP1 were effected in neuroblastoma cell lines using standard techniques. Expression was determined by qRT-PCR and confirmed at the protein level by Western blot. Metabolic viability was determined by Alamar blue assay. While protein content of CRABP1 correlated roughly with that of p75NTR in the three neuroblastoid or epithelioid human neuroblastoma cell lines studied, manipulation of p75NTR expression resulted in cell line-dependent, variable change in CRABP1 expression. Furthermore, in some cell lines, induced expression of CRABP1 in the absence of p75NTR did not alter cell sensitivity to fenretinide treatment. The effects of manipulation of p75NTR expression on CRABP1 expression and the effects of CRABP1 expression on fenretinide efficacy are therefore neuroblastoma cell line-dependent. Potentiation of the antineuroblastoma cell effects of fenretinide by p75NTR is not mediated solely through CRABP1.
Figures

Similar articles
-
p75NTR: an enhancer of fenretinide toxicity in neuroblastoma.Cancer Chemother Pharmacol. 2013 Mar;71(3):777-87. doi: 10.1007/s00280-013-2071-7. Epub 2013 Jan 13. Cancer Chemother Pharmacol. 2013. PMID: 23314735 Free PMC article.
-
p75 neurotrophin receptor and fenretinide-induced signaling in neuroblastoma.Cancer Chemother Pharmacol. 2014 Feb;73(2):271-9. doi: 10.1007/s00280-013-2355-y. Epub 2013 Nov 20. Cancer Chemother Pharmacol. 2014. PMID: 24253178 Free PMC article.
-
GADD153 and 12-lipoxygenase mediate fenretinide-induced apoptosis of neuroblastoma.Cancer Res. 2002 Sep 15;62(18):5158-67. Cancer Res. 2002. PMID: 12234979
-
Induction of GADD153 and Bak: novel molecular targets of fenretinide-induced apoptosis of neuroblastoma.Cancer Lett. 2003 Jul 18;197(1-2):157-63. doi: 10.1016/s0304-3835(03)00098-3. Cancer Lett. 2003. PMID: 12880976 Review.
-
Molecular mechanisms of fenretinide-induced apoptosis of neuroblastoma cells.Ann N Y Acad Sci. 2004 Dec;1028:81-9. doi: 10.1196/annals.1322.009. Ann N Y Acad Sci. 2004. PMID: 15650234 Review.
References
-
- Yan C., Mirnics Z. K., Portugal C. F., et al. Cholesterol biosynthesis and the pro-apoptotic effects of the p75 nerve growth factor receptor. Molecular Brain Research. 2005;139:225–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

