The Cost-Effectiveness of Continuous Erythropoiesis Receptor Activator Once Monthly versus Epoetin Thrice Weekly for Anaemia Management in Chronic Haemodialysis Patients
- PMID: 26843983
- PMCID: PMC4710935
- DOI: 10.1155/2015/189404
The Cost-Effectiveness of Continuous Erythropoiesis Receptor Activator Once Monthly versus Epoetin Thrice Weekly for Anaemia Management in Chronic Haemodialysis Patients
Abstract
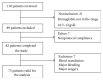
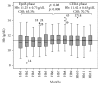
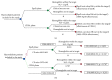
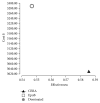
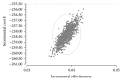
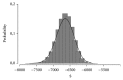
Introduction. The aim of this study was to compare the cost-effectiveness of continuous erythropoietin receptor activator (CERA) once monthly to epoetin beta (EpoB) thrice weekly to maintain haemoglobin (Hb) within the range 10.5-12 g/dL. Methods. Prospective cohort study and cost-effectiveness analysis. Chronic haemodialysis patients (CHP), being treated with EpoB, were selected for two periods of follow-up: period 1, maintaining prior treatment with EpoB, and period 2, conversion to CERA once monthly. Hb concentrations and costs were measured monthly. Health care payer perspective for one year was adopted. Results. 75 CHP completed the study, with a mean age of 52.9 ± 14.3 years. Baseline Hb was 11.14 ± 1.18 g/dL in EpoB phase and 11.46 ± 0.79 g/dL in CERA phase; we observed a significant increase in the proportion of patients successfully treated (Hb within the recommended range), 65.3% versus 70.7%, p: 0.008, and in the average effectiveness by 4% (0.55 versus 0.59). Average cost-effectiveness ratios were 6013.86 and 5173.64$, with an ICER CERA to EpoB at -6457.5$. Conclusion. Our health economic evaluation of ESA use in haemodialysis patients suggests that the use of CERA is cost-effective compared with EpoB.
Figures
Similar articles
-
The cost-utility of treating anemia with continuous erythropoietin receptor activator or Epoetin versus routine blood transfusions among chronic hemodialysis patients.Int J Nephrol Renovasc Dis. 2016 Feb 24;9:35-43. doi: 10.2147/IJNRD.S96027. eCollection 2016. Int J Nephrol Renovasc Dis. 2016. PMID: 26966386 Free PMC article.
-
Once-monthly continuous erythropoietin receptor activator (CERA) for haemoglobin maintenance in haemodialysis patients with chronic renal anaemia.Clin Kidney J. 2014 Oct;7(5):464-9. doi: 10.1093/ckj/sfu079. Epub 2014 Jul 29. Clin Kidney J. 2014. PMID: 25504109 Free PMC article.
-
Effect of conversion from ESA with shorter half-life to CERA once monthly for maintaining Hb concentration in pre-dialysis CKD patients.Kidney Blood Press Res. 2013;37(4-5):259-68. doi: 10.1159/000350151. Epub 2013 Jul 22. Kidney Blood Press Res. 2013. PMID: 24022228
-
Cost-effectiveness of continuous erythropoietin receptor activator in anemia.Clinicoecon Outcomes Res. 2014 Jul 3;6:319-30. doi: 10.2147/CEOR.S46930. eCollection 2014. Clinicoecon Outcomes Res. 2014. PMID: 25050070 Free PMC article. Review.
-
Epoetin Beta: a review of its clinical use in the treatment of anaemia in patients with cancer.Drugs. 2004;64(3):323-46. doi: 10.2165/00003495-200464030-00006. Drugs. 2004. PMID: 14871172 Review.
Cited by
-
The Cost-Effectiveness of Using Epoetin-Beta Versus Darbepoetin-Alfa for the Treatment of Anemia Among Chronic Hemodialysis Patients.Cureus. 2020 Dec 4;12(12):e11895. doi: 10.7759/cureus.11895. Cureus. 2020. PMID: 33415047 Free PMC article.
-
Cost-Minimisation Analysis of Erythropoiesis-Stimulating Agents in the Treatment of Anaemia in Dialysed Patients: A Pilot Study.Pharmacoecon Open. 2017 Sep;1(3):223-229. doi: 10.1007/s41669-017-0016-5. Pharmacoecon Open. 2017. PMID: 29441494 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

