Identification and Characterization of the V(D)J Recombination Activating Gene 1 in Long-Term Memory of Context Fear Conditioning
- PMID: 26843989
- PMCID: PMC4710954
- DOI: 10.1155/2016/1752176
Identification and Characterization of the V(D)J Recombination Activating Gene 1 in Long-Term Memory of Context Fear Conditioning
Abstract
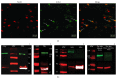
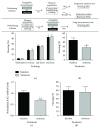
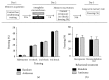
An increasing body of evidence suggests that mechanisms related to the introduction and repair of DNA double strand breaks (DSBs) may be associated with long-term memory (LTM) processes. Previous studies from our group suggested that factors known to function in DNA recombination/repair machineries, such as DNA ligases, polymerases, and DNA endonucleases, play a role in LTM. Here we report data using C57BL/6 mice showing that the V(D)J recombination-activating gene 1 (RAG1), which encodes a factor that introduces DSBs in immunoglobulin and T-cell receptor genes, is induced in the amygdala, but not in the hippocampus, after context fear conditioning. Amygdalar induction of RAG1 mRNA, measured by real-time PCR, was not observed in context-only or shock-only controls, suggesting that the context fear conditioning response is related to associative learning processes. Furthermore, double immunofluorescence studies demonstrated the neuronal localization of RAG1 protein in amygdalar sections prepared after perfusion and fixation. In functional studies, intra-amygdalar injections of RAG1 gapmer antisense oligonucleotides, given 1 h prior to conditioning, resulted in amygdalar knockdown of RAG1 mRNA and a significant impairment in LTM, tested 24 h after training. Overall, these findings suggest that the V(D)J recombination-activating gene 1, RAG1, may play a role in LTM consolidation.
Figures


Similar articles
-
An inhibitor of DNA recombination blocks memory consolidation, but not reconsolidation, in context fear conditioning.J Neurosci. 2006 May 17;26(20):5524-33. doi: 10.1523/JNEUROSCI.3050-05.2006. J Neurosci. 2006. PMID: 16707804 Free PMC article.
-
Elevated Arc/Arg 3.1 protein expression in the basolateral amygdala following auditory trace-cued fear conditioning.Neurobiol Learn Mem. 2013 Nov;106:127-33. doi: 10.1016/j.nlm.2013.07.010. Epub 2013 Jul 24. Neurobiol Learn Mem. 2013. PMID: 23891993
-
Roles of hippocampal GABA(A) and muscarinic receptors in consolidation of context memory and context-shock association in contextual fear conditioning: a double dissociation study.Neurobiol Learn Mem. 2012 Jul;98(1):17-24. doi: 10.1016/j.nlm.2012.04.004. Epub 2012 Apr 21. Neurobiol Learn Mem. 2012. PMID: 22543193
-
Systems consolidation and the content of memory.Neurobiol Learn Mem. 2013 Nov;106:365-71. doi: 10.1016/j.nlm.2013.06.001. Epub 2013 Jun 14. Neurobiol Learn Mem. 2013. PMID: 23770492 Review.
-
V(D)J recombination: RAG proteins, repair factors, and regulation.Annu Rev Biochem. 2002;71:101-32. doi: 10.1146/annurev.biochem.71.090501.150203. Epub 2001 Nov 9. Annu Rev Biochem. 2002. PMID: 12045092 Review.
Cited by
-
The Role of DNA Damage in Neural Plasticity in Physiology and Neurodegeneration.Front Cell Neurosci. 2022 Jun 23;16:836885. doi: 10.3389/fncel.2022.836885. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35813507 Free PMC article. Review.
-
Therapeutic effects of stress-programmed lymphocytes transferred to chronically stressed mice.Prog Neuropsychopharmacol Biol Psychiatry. 2016 Oct 3;70:1-7. doi: 10.1016/j.pnpbp.2016.04.010. Epub 2016 Apr 21. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 27109071 Free PMC article.
-
Cognitive neuroepigenetics: the next evolution in our understanding of the molecular mechanisms underlying learning and memory?NPJ Sci Learn. 2016;1:16014. doi: 10.1038/npjscilearn.2016.14. Epub 2016 Jul 20. NPJ Sci Learn. 2016. PMID: 27512601 Free PMC article.
-
Safeguarding genomic integrity in beta-cells: implications for beta-cell differentiation, growth, and dysfunction.Biochem Soc Trans. 2024 Oct 30;52(5):2133-2144. doi: 10.1042/BST20231519. Biochem Soc Trans. 2024. PMID: 39364746 Free PMC article. Review.
-
How do neurons live long and healthy? The mechanism of neuronal genome integrity.Front Neurosci. 2025 Mar 19;19:1552790. doi: 10.3389/fnins.2025.1552790. eCollection 2025. Front Neurosci. 2025. PMID: 40177377 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

