Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants
- PMID: 26844006
- PMCID: PMC4722072
- DOI: 10.1007/s11434-015-0929-2
Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants
Abstract
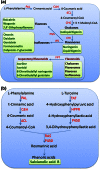
Plants synthesize and accumulate large amount of specialized (or secondary) metabolites also known as natural products, which provide a rich source for modern pharmacy. In China, plants have been used in traditional medicine for thousands of years. Recent development of molecular biology, genomics and functional genomics as well as high-throughput analytical chemical technologies has greatly promoted the research on medicinal plants. In this article, we review recent advances in the elucidation of biosynthesis of specialized metabolites in medicinal plants, including phenylpropanoids, terpenoids and alkaloids. These natural products may share a common upstream pathway to form a limited numbers of common precursors, but are characteristic in distinct modifications leading to highly variable structures. Although this review is focused on traditional Chinese medicine, other plants with a great medicinal interest or potential are also discussed. Understanding of their biosynthesis processes is critical for producing these highly value molecules at large scale and low cost in microbes and will benefit to not only human health but also plant resource conservation.
Keywords: Alkaloid; Biosynthesis; Medicinal plant; Phenylpropanoid; Terpenoid.
Figures

References
-
- Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111–129. - PubMed
-
- Qiu J. Traditional medicine: a culture in the balance. Nature. 2007;448:126–128. - PubMed
-
- Chan E, Tan M, Xin J, et al. Interactions between traditional Chinese medicines and Western therapeutics. Curr Opin Drug Discov Dev. 2010;13:50–65. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

