Incretin-like effects of small molecule trace amine-associated receptor 1 agonists
- PMID: 26844206
- PMCID: PMC4703809
- DOI: 10.1016/j.molmet.2015.09.015
Incretin-like effects of small molecule trace amine-associated receptor 1 agonists
Abstract
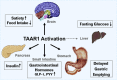
Objective: Type 2 diabetes and obesity are emerging pandemics in the 21st century creating worldwide urgency for the development of novel and safe therapies. We investigated trace amine-associated receptor 1 (TAAR1) as a novel target contributing to the control of glucose homeostasis and body weight.
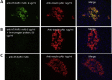
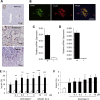
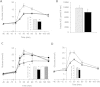
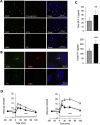
Methods: We investigated the peripheral human tissue distribution of TAAR1 by immunohistochemistry and tested the effect of a small molecule TAAR1 agonist on insulin secretion in vitro using INS1E cells and human islets and on glucose tolerance in C57Bl6, and db/db mice. Body weight effects were investigated in obese DIO mice.
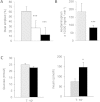
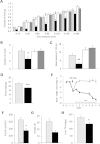
Results: TAAR1 activation by a selective small molecule agonist increased glucose-dependent insulin secretion in INS1E cells and human islets and elevated plasma PYY and GLP-1 levels in mice. In diabetic db/db mice, the TAAR1 agonist normalized glucose excursion during an oral glucose tolerance test. Sub-chronic treatment of diet-induced obese (DIO) mice with the TAAR1 agonist resulted in reduced food intake and body weight. Furthermore insulin sensitivity was improved and plasma triglyceride levels and liver triglyceride content were lower than in controls.
Conclusions: We have identified TAAR1 as a novel integrator of metabolic control, which acts on gastrointestinal and pancreatic islet hormone secretion. Thus TAAR1 qualifies as a novel and promising target for the treatment of type 2 diabetes and obesity.
Keywords: Incretin hormones; Insulin secretion; Obesity; Pancreatic β-cell; Type 2 diabetes.
Figures
References
-
- Lindemann L., Hoener M.C. A renaissance in trace amines inspired by a novel GPCR family. Trends in Pharmacological Sciences. 2005;26:274–281. - PubMed
-
- Lindemann L., Meyer C.A., Jeanneau K., Bradaia A., Ozmen L., Bluethmann H. Trace amine-associated receptor 1 modulates dopaminergic activity. Journal of Pharmacology and Experimental Therapeutics. 2008;324:948–956. - PubMed
-
- Bunzow J.R., Sonders M.S., Arttamangkul S., Harrison L.M., Zhang G., Quigley D.I. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Molecular Pharmacology. 2001;60:1181–1188. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

