Lansoprazole Upregulates Polyubiquitination of the TNF Receptor-Associated Factor 6 and Facilitates Runx2-mediated Osteoblastogenesis
- PMID: 26844285
- PMCID: PMC4703748
- DOI: 10.1016/j.ebiom.2015.11.024
Lansoprazole Upregulates Polyubiquitination of the TNF Receptor-Associated Factor 6 and Facilitates Runx2-mediated Osteoblastogenesis
Abstract
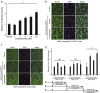
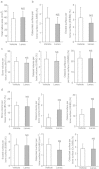
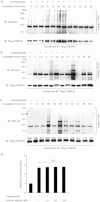
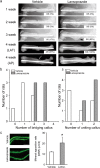
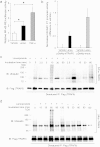
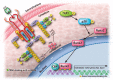
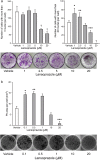
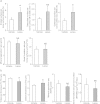
The transcription factor, runt-related transcription factor 2 (Runx2), plays a pivotal role in the differentiation of the mesenchymal stem cells to the osteochondroblast lineages. We found by the drug repositioning strategy that a proton pump inhibitor, lansoprazole, enhances nuclear accumulation of Runx2 and induces osteoblastogenesis of human mesenchymal stromal cells. Systemic administration of lansoprazole to a rat femoral fracture model increased osteoblastogenesis. Dissection of signaling pathways revealed that lansoprazole activates a noncanonical bone morphogenic protein (BMP)-transforming growth factor-beta (TGF-β) activated kinase-1 (TAK1)-p38 mitogen-activated protein kinase (MAPK) pathway. We found by in cellulo ubiquitination studies that lansoprazole enhances polyubiquitination of the TNF receptor-associated factor 6 (TRAF6) and by in vitro ubiquitination studies that the enhanced polyubiquitination of TRAF6 is attributed to the blocking of a deubiquitination enzyme, cylindromatosis (CYLD). Structural modeling and site-directed mutagenesis of CYLD demonstrated that lansoprazole tightly fits in a pocket of CYLD where the C-terminal tail of ubiquitin lies. Lansoprazole is a potential therapeutic agent for enhancing osteoblastic differentiation.
Keywords: CYLD; Drug repositioning; Lansoprazole; Osteoblastogenesis; Runx2; TRAF6.
Figures









References
-
- Abbott A. Neurologists strike gold in drug screen effort. Nature. 2002;417:109. - PubMed
-
- Bian Y., Masuda A., Matsuura T., Ito M., Okushin K., Engel A.G., Ohno K. Tannic acid facilitates expression of the polypyrimidine tract binding protein and alleviates deleterious inclusion of CHRNA1 exon P3A due to an hnRNP H-disrupting mutation in congenital myasthenic syndrome. Hum. Mol. Genet. 2009;18:1229–1237. - PMC - PubMed
-
- Brummelkamp T.R., Nijman S.M., Dirac A.M., Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

