Are Clade Specific HIV Vaccines a Necessity? An Analysis Based on Mathematical Models
- PMID: 26844286
- PMCID: PMC4703729
- DOI: 10.1016/j.ebiom.2015.11.009
Are Clade Specific HIV Vaccines a Necessity? An Analysis Based on Mathematical Models
Abstract
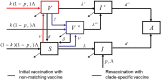
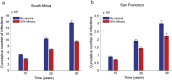
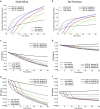
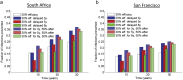
As HIV-1 envelope immune responses are critical to vaccine related protection, most candidate HIV vaccines entering efficacy trials are based upon a clade specific design. This need for clade specific vaccine prototypes markedly reduces the implementation of potentially effective HIV vaccines. We utilized a mathematical model to determine the effectiveness of immediate roll-out of a non-clade matched vaccine with reduced efficacy compared to constructing clade specific vaccines, which would take considerable time to manufacture and test in safety and efficacy trials. We simulated the HIV epidemic in San Francisco (SF) and South Africa (SA) and projected effectiveness of three vaccination strategies: i) immediate intervention with a 20-40% vaccine efficacy (VE) non-matched vaccine, ii) delayed intervention by developing a 50% VE clade-specific vaccine, and iii) immediate intervention with a non-matched vaccine replaced by a clade-specific vaccine when developed. Immediate vaccination with a non-clade matched vaccine, even with reduced efficacy, would prevent thousands of new infections in SF and millions in SA over 30 years. Vaccination with 50% VE delayed for five years needs six and 12 years in SA to break-even with immediate 20 and 30% VE vaccination, respectively, while not able to surpass the impact of immediate 40% VE vaccination over 30 years. Replacing a 30% VE with a 50% VE vaccine after 5 years reduces the HIV acquisition by 5% compared to delayed vaccination. The immediate use of an HIV vaccine with reduced VE in high risk communities appears desirable over a short time line but higher VE should be the pursued to achieve strong long-term impact. Our analysis illustrates the importance of developing surrogate markers (correlates of protection) to allow bridging types of immunogenicity studies to support more rapid assessment of clade specific vaccines.
Keywords: ART, antiretroviral therapy; HIV epidemic; HIV prevention; HIV, human immunodeficiency virus; Intervention effectiveness; Mathematical modeling; NHP, non-human primates; SA, South Africa; SF, San Francisco; VE, vaccine efficacy; Vaccine efficacy.
Figures

Comment in
-
Using the Tools we Have: Low-efficacy Vaccines and HIV.EBioMedicine. 2015 Dec 12;2(12):1867-8. doi: 10.1016/j.ebiom.2015.12.007. eCollection 2015 Dec. EBioMedicine. 2015. PMID: 26844264 Free PMC article. No abstract available.
References
-
- Andersson K.M., Stover J. The potential impact of a moderately effective HIV vaccine with rapidly waning protection in South Africa and Thailand. Vaccine. 2011;29(36):6092–6099. - PubMed
-
- Anon. The 2010 scientific strategic plan of the Global HIV Vaccine Enterprise. Nat. Med. 2010;16(9):981–989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

