Seasonal reproductive endothermy in tegu lizards
- PMID: 26844295
- PMCID: PMC4737272
- DOI: 10.1126/sciadv.1500951
Seasonal reproductive endothermy in tegu lizards
Abstract
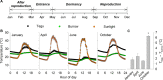
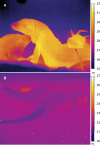
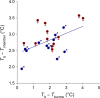
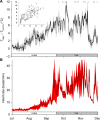
With some notable exceptions, small ectothermic vertebrates are incapable of endogenously sustaining a body temperature substantially above ambient temperature. This view was challenged by our observations of nighttime body temperatures sustained well above ambient (up to 10°C) during the reproductive season in tegu lizards (~2 kg). This led us to hypothesize that tegus have an enhanced capacity to augment heat production and heat conservation. Increased metabolic rates and decreased thermal conductance are the same mechanisms involved in body temperature regulation in those vertebrates traditionally acknowledged as "true endotherms": the birds and mammals. The appreciation that a modern ectotherm the size of the earliest mammals can sustain an elevated body temperature through metabolic rates approaching that of endotherms enlightens the debate over endothermy origins, providing support for the parental care model of endothermy, but not for the assimilation capacity model of endothermy. It also indicates that, contrary to prevailing notions, ectotherms can engage in facultative endothermy, providing a physiological analog in the evolutionary transition to true endothermy.
Keywords: Evolution; Life sciences; animal science; breeding season; endothermy; lizard; parental care; reptile; thermogenesis.
Figures
Comment in
-
Evolution: A lizard that generates heat.Nature. 2016 Jan 28;529(7587):470-2. doi: 10.1038/529470a. Nature. 2016. PMID: 26819039 No abstract available.
Similar articles
-
Reptile thermogenesis and the origins of endothermy.Zoology (Jena). 2016 Oct;119(5):403-405. doi: 10.1016/j.zool.2016.03.001. Epub 2016 Mar 7. Zoology (Jena). 2016. PMID: 27133734
-
Mitochondrial function in skeletal muscle contributes to reproductive endothermy in tegu lizards (Salvator merianae).Acta Physiol (Oxf). 2024 Jul;240(7):e14162. doi: 10.1111/apha.14162. Epub 2024 May 13. Acta Physiol (Oxf). 2024. PMID: 38741523
-
The evolution of endothermy and its diversity in mammals and birds.Physiol Biochem Zool. 2004 Nov-Dec;77(6):982-97. doi: 10.1086/425188. Physiol Biochem Zool. 2004. PMID: 15674771 Review.
-
A phenology of the evolution of endothermy in birds and mammals.Biol Rev Camb Philos Soc. 2017 May;92(2):1213-1240. doi: 10.1111/brv.12280. Epub 2016 May 7. Biol Rev Camb Philos Soc. 2017. PMID: 27154039 Review.
-
Metabolic heat production and thermal conductance are mass-independent adaptations to thermal environment in birds and mammals.Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):15934-9. doi: 10.1073/pnas.1521662112. Epub 2015 Dec 14. Proc Natl Acad Sci U S A. 2015. PMID: 26668359 Free PMC article.
Cited by
-
Muscle Non-shivering Thermogenesis and Its Role in the Evolution of Endothermy.Front Physiol. 2017 Nov 9;8:889. doi: 10.3389/fphys.2017.00889. eCollection 2017. Front Physiol. 2017. PMID: 29170642 Free PMC article. Review.
-
Revisiting concepts of thermal physiology: understanding negative feedback and set-point in mammals, birds, and lizards.Biol Rev Camb Philos Soc. 2025 Jun;100(3):1317-1346. doi: 10.1111/brv.70002. Epub 2025 Feb 6. Biol Rev Camb Philos Soc. 2025. PMID: 39912218 Free PMC article. Review.
-
Subtropical hibernation in juvenile tegu lizards (Salvator merianae): insights from intestine redox dynamics.Sci Rep. 2018 Jun 19;8(1):9368. doi: 10.1038/s41598-018-27263-x. Sci Rep. 2018. PMID: 29921981 Free PMC article.
-
The evolution of mechanisms involved in vertebrate endothermy.Philos Trans R Soc Lond B Biol Sci. 2020 Mar 2;375(1793):20190136. doi: 10.1098/rstb.2019.0136. Epub 2020 Jan 13. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 31928191 Free PMC article. Review.
-
Seasonal variation of behavioural thermoregulation in a fossorial salamander (Ambystoma maculatum).R Soc Open Sci. 2024 Sep 4;11(9):240537. doi: 10.1098/rsos.240537. eCollection 2024 Sep. R Soc Open Sci. 2024. PMID: 39233724 Free PMC article.
References
-
- Kemp T. S., The origin of mammalian endothermy: a paradigm for the evolution of complex biological structure. Zool. J. Linn. Soc. 147, 473–488 (2006).
-
- Hayes J. P., Garland T., The evolution of endothermy: Testing the aerobic capacity model. Evolution 49, 836–847 (1995). - PubMed
-
- Bennett A. F., Ruben J. A., Endothermy and activity in vertebrates. Science 206, 649–654 (1979). - PubMed
-
- Crompton A. W., Taylor C. R., Jagger J. A., Evolution of homeothermy in mammals. Nature 272, 333–336 (1978). - PubMed
-
- McNab B. K., The evolution of endothermy in the phylogeny of mammals. Am. Nat. 112, 1–21 (1978).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

