Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche
- PMID: 26844426
- PMCID: PMC4777643
- DOI: 10.1016/j.actbio.2016.01.043
Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche
Abstract
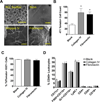
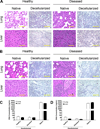
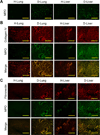
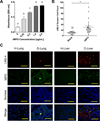
Metastatic tumor cells colonize the pre-metastatic niche, which is a complex microenvironment consisting partially of extracellular matrix (ECM) proteins. We sought to identify and validate novel contributors to tumor cell colonization using ECM-coated poly(ε-caprolactone) (PCL) scaffolds as mimics of the pre-metastatic niche. Utilizing orthotopic breast cancer mouse models, fibronectin and collagen IV-coated scaffolds implanted in the subcutaneous space captured colonizing tumor cells, showing a greater than 2-fold increase in tumor cell accumulation at the implant site compared to uncoated scaffolds. As a strategy to identify additional ECM colonization contributors, decellularized matrix (DCM) from lungs and livers containing metastatic tumors were characterized. In vitro, metastatic cell adhesion was increased on DCM coatings from diseased organs relative to healthy DCM. Furthermore, in vivo implantations of diseased DCM-coated scaffolds had increased tumor cell colonization relative to healthy DCM coatings. Mass-spectrometry proteomics was performed on healthy and diseased DCM to identify candidates associated with colonization. Myeloperoxidase was identified as abundantly present in diseased organs and validated as a contributor to colonization using myeloperoxidase-coated scaffold implants. This work identified novel ECM proteins associated with colonization using decellularization and proteomics techniques and validated candidates using a scaffold to mimic the pre-metastatic niche.
Statement of significance: The pre-metastatic niche consists partially of ECM proteins that promote metastatic cell colonization to a target organ. We present a biomaterials-based approach to mimic this niche and identify ECM mediators of colonization. Using murine breast cancer models, we implanted microporous PCL scaffolds to recruit colonizing tumor cells in vivo. As a strategy to modulate colonization, we coated scaffolds with various ECM proteins, including decellularized lung and liver matrix from tumor-bearing mice. After characterizing the organ matrices using proteomics, myeloperoxidase was identified as an ECM protein contributing to colonization and validated using our scaffold. Our scaffold provides a platform to identify novel contributors to colonization and allows for the capture of colonizing tumor cells for a variety of downstream clinical applications.
Keywords: Colonization; Extracellular matrix; Metastasis; Organ decellularization; Pre-metastatic niche.
Copyright © 2016 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

