Prognostic Value of Plasma Epstein-Barr Virus DNA for Local and Regionally Advanced Nasopharyngeal Carcinoma Treated With Cisplatin-Based Concurrent Chemoradiotherapy in Intensity-Modulated Radiotherapy Era
- PMID: 26844482
- PMCID: PMC4748899
- DOI: 10.1097/MD.0000000000002642
Prognostic Value of Plasma Epstein-Barr Virus DNA for Local and Regionally Advanced Nasopharyngeal Carcinoma Treated With Cisplatin-Based Concurrent Chemoradiotherapy in Intensity-Modulated Radiotherapy Era
Abstract
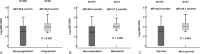
This study aimed to evaluate the prognostic value of plasma Epstein-Barr Virus DNA (EBV DNA) for local and regionally advanced nasopharyngeal carcinoma (NPC) patients treated with concurrent chemoradiotherapy in intensity-modulated radiotherapy (IMRT) era.In this observational study, 404 nonmetastatic local and regionally advanced NPC patients treated with IMRT and cisplatin-based concurrent chemotherapy were recruited. Blood samples were collected before treatment for examination of plasma EBV DNA levels. We evaluated the association of pretreatment plasma EBV DNA levels with progression-free survival rate (PFS), distant metastasis-free survival rate (DMFS), and overall survival rate (OS).Compared to patients with an EBV DNA level < 4000 copies/mL, patients with an EBV DNA ≥ 4000 copies/mL had a lower rate of 3-year PFS (76%, 95% CI [68-84]) versus (93%, 95% CI [90-96], P < 0.001), DMFS (83%, 95% CI [76-89]) versus (97%, 95% CI [94-99], P < 0.001), and OS (85%, 95% CI [78-92]) versus (98%, 95% CI [95-100], P < 0.001). Multivariate analysis showed that pretreatment EBV DNA levels (HR = 3.324, 95% CI, 1.80-6.138, P < 0.001) and clinical stage (HR = 1.878, 95% CI, 1.036-3.404, P = 0.038) were the only independent factor associated with PFS, pretreatment EBV DNA level was the only significant factor to predict DMFS (HR = 6.292, 95% CI, 2.647-14.956, P < 0.001), and pretreatment EBV DNA levels (HR = 3.753, 95% CI, 1.701-8.284, P < 0.001) and clinical stage (HR = 2.577, 95% CI, 1.252-5.050, P = 0.010) were significantly associated with OS. In subgroup analysis, higher plasma EBV DNA levels still predicted a worse PFS, DMFS, and OS for the patients stage III or stage IVa-b, compared with those with low EBV DNA levels.Elevated plasma EBV DNA was still effective prognostic biomarker for local and regionally advanced NPC patients treated with IMRT and cisplatin-based concurrent chemotherapy. Future ramdomized clinical trials are needed to further evaluate whether plasma EBV DNA levels could be applied to guide concurrent chemotherapy regimen for local and regionally advanced NPC patients.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Wee JT, Ha TC, Loong SL, et al. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer 2010; 29:517–526. - PubMed
-
- Ferlay JS, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No.11. 2013; Lyon: International Agency for Research on Cancer, 2013–2012–2012, 2014–2001–2018.
-
- Langendijk JA, Leemans CR, Buter J, et al. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol 2004; 22:4604–4612. - PubMed
-
- Huncharek M, Kupelnick B. Combined chemoradiation versus radiation therapy alone in locally advanced nasopharyngeal carcinoma: results of a meta-analysis of 1,528 patients from six randomized trials. Am J Clin Oncol 2002; 25:219–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

