Efficacy and Safety of Bisphosphonates for Low Bone Mineral Density After Kidney Transplantation: A Meta-Analysis
- PMID: 26844505
- PMCID: PMC4748922
- DOI: 10.1097/MD.0000000000002679
Efficacy and Safety of Bisphosphonates for Low Bone Mineral Density After Kidney Transplantation: A Meta-Analysis
Abstract
In patients with low bone mineral density (BMD) after kidney transplantation, the role of bisphosphonates remains unclear. We performed a systematic review and meta-analysis to investigate the efficacy and safety of bisphosphonates.We retrieved trials from PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception through May 2015. Only randomized controlled trials that compared bisphosphonate-treated and control groups of patients with low bone mineral density after kidney transplantation were included. The primary outcomes were the percent change in BMD, the absolute change in BMD, and the BMD at the end of study at the lumbar spine. The results were expressed as the mean difference (MD) or relative risk (RR) with the 95% confidence interval (CI). We used a random-effects model to pool the outcomes.We included 17 randomized controlled trials with 1067 patients. Only 1 included trial was found to be at low risk of bias. The rest of the included studies were found to have high to uncertain risk of bias. Compared with the control group, those who received bisphosphonates had a significant increase in percent change in BMD (mean difference [MD] = 5.51, 95% confidence interval [CI] 3.22-7.79, P < 0.00001) and absolute change in BMD (MD = 0.05, 95% CI 0.04-0.05, P < 0.00001), but a nonsignificant increase in BMD at the end of the study (MD = 0.02, 95% CI -0.01 to 0.05, P = 0.25) at the lumbar spine. Bisphosphonates resulted in a significant improvement in percent change in BMD (MD = 4.95, 95% CI 2.57-7.33, P < 0.0001), but a nonsignificant improvement in absolute change in BMD (MD = 0.03, 95% CI -0.00 to 0.06, P = 0.07) and BMD at the end of the study (MD = -0.01, 95% CI -0.04 to 0.02, P = 0.40) at the femoral neck. No significant differences were found in vertebral fractures, nonvertebral fractures, adverse events, and gastrointestinal adverse events.Bisphosphonates appear to have a beneficial effect on BMD at the lumbar spine and do not significantly decrease fracture events in recipients. However, the results should be interpreted cautiously due to the lack of robustness and the heterogeneity among studies.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

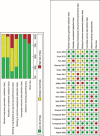




References
-
- Lim DS, Kee TY, Fook-Chong S, et al. Prevalence and patterns of bone loss in the first year after renal transplant in South East Asian patients. Transplantation 2011; 92:557–563. - PubMed
-
- Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transpl 2015; 15 (S2):1–34. - PubMed
-
- Hariharan S. Long-term kidney transplant survival. Am J Kidney Dis 2001; 38: suppl 6:S44–S50. - PubMed
-
- Torregrosa JV, Moreno A, Gutierrez A, et al. Alendronate for treatment of renal transplant patients with osteoporosis. Transplant Proc 2003; 35:1393–1395. - PubMed
-
- Grotz WH, Mundinger FA, Gugel B, et al. Bone fracture and osteodensitometry with dual energy X-ray absorptiometry in kidney transplant recipients. Transplantation 1994; 58:912–915. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

