A Missense LRRK2 Variant Is a Risk Factor for Excessive Inflammatory Responses in Leprosy
- PMID: 26844546
- PMCID: PMC4742274
- DOI: 10.1371/journal.pntd.0004412
A Missense LRRK2 Variant Is a Risk Factor for Excessive Inflammatory Responses in Leprosy
Abstract
Background: Depending on the epidemiological setting, a variable proportion of leprosy patients will suffer from excessive pro-inflammatory responses, termed type-1 reactions (T1R). The LRRK2 gene encodes a multi-functional protein that has been shown to modulate pro-inflammatory responses. Variants near the LRRK2 gene have been associated with leprosy in some but not in other studies. We hypothesized that LRRK2 was a T1R susceptibility gene and that inconsistent association results might reflect different proportions of patients with T1R in the different sample settings. Hence, we evaluated the association of LRRK2 variants with T1R susceptibility.
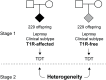
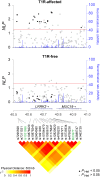
Methodology: An association scan of the LRRK2 locus was performed using 156 single-nucleotide polymorphisms (SNPs). Evidence of association was evaluated in two family-based samples: A set of T1R-affected and a second set of T1R-free families. Only SNPs significant for T1R-affected families with significant evidence of heterogeneity relative to T1R-free families were considered T1R-specific. An expression quantitative trait locus (eQTL) analysis was applied to evaluate the impact of T1R-specific SNPs on LRRK2 gene transcriptional levels.
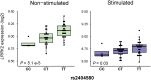
Principal findings: A total of 18 T1R-specific variants organized in four bins were detected. The core SNP capturing the T1R association was the LRRK2 missense variant M2397T (rs3761863) that affects LRRK2 protein turnover. Additionally, a bin of nine SNPs associated with T1R were eQTLs for LRRK2 in unstimulated whole blood cells but not after exposure to Mycobacterium leprae antigen.
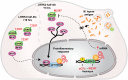
Significance: The results support a preferential association of LRRK2 variants with T1R. LRRK2 involvement in T1R is likely due to a pathological pro-inflammatory loop modulated by LRRK2 availability. Interestingly, the M2397T variant was reported in association with Crohn's disease with the same risk allele as in T1R suggesting common inflammatory mechanism in these two distinct diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Organization WH. Global leprosy update, 2014: need for early case detection. Wkly Epidemiol Rec. 2015;90(36):461–74. . - PubMed
-
- Lienhardt C, Fine PE. Type 1 reaction, neuritis and disability in leprosy. What is the current epidemiological situation? Lepr Rev. 1994;65(1):9–33. . - PubMed
-
- Fava V, Orlova M, Cobat A, Alcais A, Mira M, Schurr E. Genetics of leprosy reactions: an overview. Mem Inst Oswaldo Cruz. 2012;107 Suppl 1:132–42. Epub 2013/01/11. doi: S0074-02762012000900020 [pii]. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

