Assembly mechanism of Trypanosoma brucei BILBO1 at the flagellar pocket collar
- PMID: 26844754
- PMCID: PMC4594465
- DOI: 10.4161/19420889.2014.992739
Assembly mechanism of Trypanosoma brucei BILBO1 at the flagellar pocket collar
Abstract
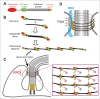
The flagellar pocket is a bulb-like invagination of the plasma membrane that encloses the base of the single flagellum in trypanosomes. It is the site of all endo- and exocytic activity in the parasite and has thus been proposed to be a therapeutic target. At the neck of the flagellar pocket is an electron-dense cytoskeletal structure named the flagellar pocket collar. The protein BILBO1 was the first characterized and remains the only known component of the flagellar pocket collar, with essential functions in the biogenesis of both the flagellar pocket and flagellar pocket collar. We recently reported that the filamentous assembly of Trypanosoma brucei BILBO1 (TbBILBO1) is mediated by its central coiled coil domain and C-terminal leucine zipper. Here, we discuss how TbBILBO1 might assemble at the flagellar pocket collar in T. brucei.
Keywords: BILBO1; Trypanosoma brucei; cytoskeleton; flagellar pocket; flagellar pocket collar; parasite; protein assembly.
Figures

References
-
- Welburn SC, Maudlin I. Priorities for the elimination of sleeping sickness. Adv Parasitol 2012; 79: p. 299-337; PMID:22726645 - PubMed
-
- Ralston KS, Kabututu ZP, Melehani JH, Oberholzer M, Hill KL. The Trypanosoma brucei flagellum: moving parasites in new directions. Annu Rev Microbiol 2009; 63: p. 335-62; PMID:19575562; http://dx.doi.org/ 10.1146/annurev.micro.091208.073353 - DOI - PMC - PubMed
-
- Field MC. Carrington M. The trypanosome flagellar pocket. Nat Rev Microbiol, 2009. 7(11): p. 775-86; PMID:19806154; http://dx.doi.org/ 10.1038/nrmicro2221 - DOI - PubMed
-
- Lacomble S, Vaughan S, Gadelha C, Morphew MK, Shaw MK, McIntosh JR, Gull K. Three-dimensional cellular architecture of the flagellar pocket and associated cytoskeleton in trypanosomes revealed by electron microscope tomography. J Cell Sci 2009; 122(Pt 8): p. 1081-90; PMID:19299460; http://dx.doi.org/ 10.1242/jcs.045740 - DOI - PMC - PubMed
-
- Bonhivers M, Nowacki S, Landrein N, Robinson DR. Biogenesis of the trypanosome endo-exocytotic organelle is cytoskeleton mediated. PLoS Biol 2008; 6(5): p. e105; PMID:18462016; http://dx.doi.org/ 10.1371/journal.pbio.0060105 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
