Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism
- PMID: 26844829
- PMCID: PMC4742785
- DOI: 10.1016/j.neuron.2015.12.023
Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism
Abstract
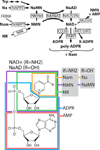
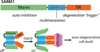
Wallerian axon degeneration is a form of programmed subcellular death that promotes axon breakdown in disease and injury. Active degeneration requires SARM1 and MAP kinases, including DLK, while the NAD+ synthetic enzyme NMNAT2 prevents degeneration. New studies reveal that these pathways cooperate in a locally mediated axon destruction program, with NAD+ metabolism playing a central role. Here, we review the biology of Wallerian-type axon degeneration and discuss the most recent findings, with special emphasis on critical signaling events and their potential as therapeutic targets for axonopathy.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

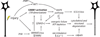
Similar articles
-
SARM1 activation triggers axon degeneration locally via NAD⁺ destruction.Science. 2015 Apr 24;348(6233):453-7. doi: 10.1126/science.1258366. Epub 2015 Apr 23. Science. 2015. PMID: 25908823 Free PMC article.
-
Mitochondrial impairment activates the Wallerian pathway through depletion of NMNAT2 leading to SARM1-dependent axon degeneration.Neurobiol Dis. 2020 Feb;134:104678. doi: 10.1016/j.nbd.2019.104678. Epub 2019 Nov 15. Neurobiol Dis. 2020. PMID: 31740269 Free PMC article.
-
Protective effects of NAMPT or MAPK inhibitors and NaR on Wallerian degeneration of mammalian axons.Neurobiol Dis. 2022 Sep;171:105808. doi: 10.1016/j.nbd.2022.105808. Epub 2022 Jun 30. Neurobiol Dis. 2022. PMID: 35779777 Free PMC article.
-
The SARM1 axon degeneration pathway: control of the NAD+ metabolome regulates axon survival in health and disease.Curr Opin Neurobiol. 2020 Aug;63:59-66. doi: 10.1016/j.conb.2020.02.012. Epub 2020 Apr 17. Curr Opin Neurobiol. 2020. PMID: 32311648 Free PMC article. Review.
-
The chemical biology of NAD+ regulation in axon degeneration.Curr Opin Chem Biol. 2022 Aug;69:102176. doi: 10.1016/j.cbpa.2022.102176. Epub 2022 Jul 1. Curr Opin Chem Biol. 2022. PMID: 35780654 Free PMC article. Review.
Cited by
-
Multifaceted roles of SARM1 in axon degeneration and signaling.Front Cell Neurosci. 2022 Aug 25;16:958900. doi: 10.3389/fncel.2022.958900. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36090788 Free PMC article. Review.
-
Wnd/DLK Is a Critical Target of FMRP Responsible for Neurodevelopmental and Behavior Defects in the Drosophila Model of Fragile X Syndrome.Cell Rep. 2019 Sep 3;28(10):2581-2593.e5. doi: 10.1016/j.celrep.2019.08.001. Cell Rep. 2019. PMID: 31484070 Free PMC article.
-
Degeneration of Injured Axons and Dendrites Requires Restraint of a Protective JNK Signaling Pathway by the Transmembrane Protein Raw.J Neurosci. 2019 Oct 23;39(43):8457-8470. doi: 10.1523/JNEUROSCI.0016-19.2019. Epub 2019 Sep 6. J Neurosci. 2019. PMID: 31492772 Free PMC article.
-
Cellular Compartmentation and the Redox/Nonredox Functions of NAD.Antioxid Redox Signal. 2019 Sep 20;31(9):623-642. doi: 10.1089/ars.2018.7722. Epub 2019 Mar 26. Antioxid Redox Signal. 2019. PMID: 30784294 Free PMC article.
-
Untangling the Tauopathy for Alzheimer's disease and parkinsonism.J Biomed Sci. 2018 Jul 10;25(1):54. doi: 10.1186/s12929-018-0457-x. J Biomed Sci. 2018. PMID: 29991349 Free PMC article. Review.
References
-
- Aksoy P, White Ta, Thompson M, Chini EN. Regulation of intracellular levels of NAD: A novel role for CD38. Biochem. Biophys. Res. Commun. 2006;345:1386–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

