A Transient Translaminar GABAergic Interneuron Circuit Connects Thalamocortical Recipient Layers in Neonatal Somatosensory Cortex
- PMID: 26844833
- PMCID: PMC4742537
- DOI: 10.1016/j.neuron.2016.01.015
A Transient Translaminar GABAergic Interneuron Circuit Connects Thalamocortical Recipient Layers in Neonatal Somatosensory Cortex
Abstract
GABAergic activity is thought to influence developing neocortical sensory circuits. Yet the late postnatal maturation of local layer (L)4 circuits suggests alternate sources of GABAergic control in nascent thalamocortical networks. We show that a population of L5b, somatostatin (SST)-positive interneuron receives early thalamic synaptic input and, using laser-scanning photostimulation, identify an early transient circuit between these cells and L4 spiny stellates (SSNs) that disappears by the end of the L4 critical period. Sensory perturbation disrupts the transition to a local GABAergic circuit, suggesting a link between translaminar and local control of SSNs. Conditional silencing of SST+ interneurons or conversely biasing the circuit toward local inhibition by overexpression of neuregulin-1 type 1 results in an absence of early L5b GABAergic input in mutants and delayed thalamic innervation of SSNs. These data identify a role for L5b SST+ interneurons in the control of SSNs in the early postnatal neocortex.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


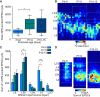
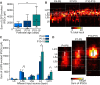

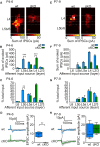
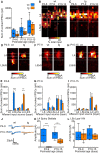
Similar articles
-
Distinct Translaminar Glutamatergic Circuits to GABAergic Interneurons in the Neonatal Auditory Cortex.Cell Rep. 2017 May 9;19(6):1141-1150. doi: 10.1016/j.celrep.2017.04.044. Cell Rep. 2017. PMID: 28494864 Free PMC article.
-
Brain-Wide Maps of Synaptic Input to Cortical Interneurons.J Neurosci. 2016 Apr 6;36(14):4000-9. doi: 10.1523/JNEUROSCI.3967-15.2016. J Neurosci. 2016. PMID: 27053207 Free PMC article.
-
Early Somatostatin Interneuron Connectivity Mediates the Maturation of Deep Layer Cortical Circuits.Neuron. 2016 Feb 3;89(3):521-35. doi: 10.1016/j.neuron.2015.11.020. Neuron. 2016. PMID: 26844832 Free PMC article.
-
Fast-spike interneurons and feedforward inhibition in awake sensory neocortex.Cereb Cortex. 2003 Jan;13(1):25-32. doi: 10.1093/cercor/13.1.25. Cereb Cortex. 2003. PMID: 12466212 Review.
-
Spike timing and synaptic dynamics at the awake thalamocortical synapse.Prog Brain Res. 2005;149:91-105. doi: 10.1016/S0079-6123(05)49008-1. Prog Brain Res. 2005. PMID: 16226579 Review.
Cited by
-
A Late Critical Period for Frequency Modulated Sweeps in the Mouse Auditory System.Cereb Cortex. 2020 Apr 14;30(4):2586-2599. doi: 10.1093/cercor/bhz262. Cereb Cortex. 2020. PMID: 31800018 Free PMC article.
-
Thalamocortical control of cell-type specificity drives circuits for processing whisker-related information in mouse barrel cortex.Nat Commun. 2023 Sep 28;14(1):6077. doi: 10.1038/s41467-023-41749-x. Nat Commun. 2023. PMID: 37770450 Free PMC article.
-
Circuitry Underlying Experience-Dependent Plasticity in the Mouse Visual System.Neuron. 2020 Apr 8;106(1):21-36. doi: 10.1016/j.neuron.2020.01.031. Neuron. 2020. PMID: 32272065 Free PMC article. Review.
-
Developmental loss of ErbB4 in PV interneurons disrupts state-dependent cortical circuit dynamics.Mol Psychiatry. 2023 Jul;28(7):3133-3143. doi: 10.1038/s41380-023-02066-3. Epub 2023 Apr 17. Mol Psychiatry. 2023. PMID: 37069344 Free PMC article.
-
Perinatal serotonin signalling dynamically influences the development of cortical GABAergic circuits with consequences for lifelong sensory encoding.Nat Commun. 2025 Jun 4;16(1):5203. doi: 10.1038/s41467-025-59659-5. Nat Commun. 2025. PMID: 40467568 Free PMC article.
References
-
- Agmon A., Connors B.W. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Allendoerfer K.L., Shatz C.J. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu. Rev. Neurosci. 1994;17:185–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

