Sensorimotor Transformations Underlying Variability in Song Intensity during Drosophila Courtship
- PMID: 26844835
- PMCID: PMC5047376
- DOI: 10.1016/j.neuron.2015.12.035
Sensorimotor Transformations Underlying Variability in Song Intensity during Drosophila Courtship
Abstract
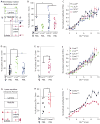
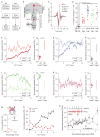
Diverse animal species, from insects to humans, utilize acoustic signals for communication. Studies of the neural basis for song or speech production have focused almost exclusively on the generation of spectral and temporal patterns, but animals can also adjust acoustic signal intensity when communicating. For example, humans naturally regulate the loudness of speech in accord with a visual estimate of receiver distance. The underlying mechanisms for this ability remain uncharacterized in any system. Here, we show that Drosophila males modulate courtship song amplitude with female distance, and we investigate each stage of the sensorimotor transformation underlying this behavior, from the detection of particular visual stimulus features and the timescales of sensory processing to the modulation of neural and muscle activity that generates song. Our results demonstrate an unanticipated level of control in insect acoustic communication and uncover novel computations and mechanisms underlying the regulation of acoustic signal intensity.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





References
-
- Alexander R. Aggressiveness, Territoriality, and Sexual Behavior in Field Crickets (Orthoptera: Gryllidae) Behaviour. 1961;17:130–223.
-
- Bath D, Stowers J, Hörmann D, Poehlmann A, Dickson B, Straw A. FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nature Methods. 2014;11 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

