A Randomized Trial Comparing Skin Antiseptic Agents at Cesarean Delivery
- PMID: 26844840
- PMCID: PMC4777327
- DOI: 10.1056/NEJMoa1511048
A Randomized Trial Comparing Skin Antiseptic Agents at Cesarean Delivery
Abstract
Background: Preoperative skin antisepsis has the potential to decrease the risk of surgical-site infection. However, evidence is limited to guide the choice of antiseptic agent at cesarean delivery, which is the most common major surgical procedure among women in the United States.
Methods: In this single-center, randomized, controlled trial, we evaluated whether the use of chlorhexidine-alcohol for preoperative skin antisepsis was superior to the use of iodine-alcohol for the prevention of surgical-site infection after cesarean delivery. We randomly assigned patients undergoing cesarean delivery to skin preparation with either chlorhexidine-alcohol or iodine-alcohol. The primary outcome was superficial or deep surgical-site infection within 30 days after cesarean delivery, on the basis of definitions from the Centers for Disease Control and Prevention.
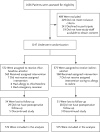
Results: From September 2011 through June 2015, a total of 1147 patients were enrolled; 572 patients were assigned to chlorhexidine-alcohol and 575 to iodine-alcohol. In an intention-to-treat analysis, surgical-site infection was diagnosed in 23 patients (4.0%) in the chlorhexidine-alcohol group and in 42 (7.3%) in the iodine-alcohol group (relative risk, 0.55; 95% confidence interval, 0.34 to 0.90; P=0.02). The rate of superficial surgical-site infection was 3.0% in the chlorhexidine-alcohol group and 4.9% in the iodine-alcohol group (P=0.10); the rate of deep infection was 1.0% and 2.4%, respectively (P=0.07). The frequency of adverse skin reactions was similar in the two groups.
Conclusions: The use of chlorhexidine-alcohol for preoperative skin antisepsis resulted in a significantly lower risk of surgical-site infection after cesarean delivery than did the use of iodine-alcohol. (Funded by the National Institutes of Health and Washington University School of Medicine in St. Louis; ClinicalTrials.gov number, NCT01472549.).
Figures

References
-
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007;165:1–209. - PubMed
-
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births final data for 2013. Hyattsville, MD: National Center for Health Statistics; Jan 15, 2015. - PubMed
-
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3):267.e1–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
