Primary visual cortex shows laminar-specific and balanced circuit organization of excitatory and inhibitory synaptic connectivity
- PMID: 26844927
- PMCID: PMC4818606
- DOI: 10.1113/JP271891
Primary visual cortex shows laminar-specific and balanced circuit organization of excitatory and inhibitory synaptic connectivity
Abstract
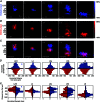
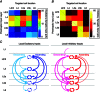
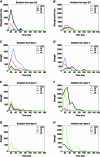
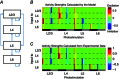
Key points: Using functional mapping assays, we conducted a quantitative assessment of both excitatory and inhibitory synaptic laminar connections to excitatory neurons in layers 2/3-6 of the mouse visual cortex (V1). Laminar-specific synaptic wiring diagrams of excitatory neurons were constructed on the basis of circuit mapping. The present study reveals that that excitatory and inhibitory synaptic connectivity is spatially balanced across excitatory neuronal networks in V1.
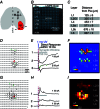
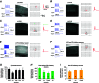
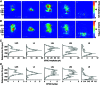
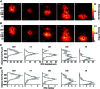
Abstract: In the mammalian neocortex, excitatory neurons provide excitation in both columnar and laminar dimensions, which is modulated further by inhibitory neurons. However, our understanding of intracortical excitatory and inhibitory synaptic inputs in relation to principal excitatory neurons remains incomplete, and it is unclear how local excitatory and inhibitory synaptic connections to excitatory neurons are spatially organized on a layer-by-layer basis. In the present study, we combined whole cell recordings with laser scanning photostimulation via glutamate uncaging to map excitatory and inhibitory synaptic inputs to single excitatory neurons throughout cortical layers 2/3-6 in the mouse primary visual cortex (V1). We find that synaptic input sources of excitatory neurons span the radial columns of laminar microcircuits, and excitatory neurons in different V1 laminae exhibit distinct patterns of layer-specific organization of excitatory inputs. Remarkably, the spatial extent of inhibitory inputs of excitatory neurons for a given layer closely mirrors that of their excitatory input sources, indicating that excitatory and inhibitory synaptic connectivity is spatially balanced across excitatory neuronal networks. Strong interlaminar inhibitory inputs are found, particularly for excitatory neurons in layers 2/3 and 5. This differs from earlier studies reporting that inhibitory cortical connections to excitatory neurons are generally localized within the same cortical layer. On the basis of the functional mapping assays, we conducted a quantitative assessment of both excitatory and inhibitory synaptic laminar connections to excitatory cells at single cell resolution, establishing precise layer-by-layer synaptic wiring diagrams of excitatory neurons in the visual cortex.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures

Similar articles
-
Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons.Nat Neurosci. 2000 Jul;3(7):701-7. doi: 10.1038/76656. Nat Neurosci. 2000. PMID: 10862703
-
Vision loss shifts the balance of feedforward and intracortical circuits in opposite directions in mouse primary auditory and visual cortices.J Neurosci. 2015 Jun 10;35(23):8790-801. doi: 10.1523/JNEUROSCI.4975-14.2015. J Neurosci. 2015. PMID: 26063913 Free PMC article.
-
Layer-specific excitation/inhibition balances during neuronal synchronization in the visual cortex.J Physiol. 2018 May 1;596(9):1639-1657. doi: 10.1113/JP274986. Epub 2018 Jan 24. J Physiol. 2018. PMID: 29313982 Free PMC article.
-
Salient features of synaptic organisation in the cerebral cortex.Brain Res Brain Res Rev. 1998 May;26(2-3):113-35. doi: 10.1016/s0165-0173(97)00061-1. Brain Res Brain Res Rev. 1998. PMID: 9651498 Review.
-
Mapping functional connectivity in barrel-related columns reveals layer- and cell type-specific microcircuits.Brain Struct Funct. 2007 Sep;212(2):107-19. doi: 10.1007/s00429-007-0147-z. Epub 2007 Jun 26. Brain Struct Funct. 2007. PMID: 17717691 Review.
Cited by
-
Time-delimited signaling of MET receptor tyrosine kinase regulates cortical circuit development and critical period plasticity.Mol Psychiatry. 2021 Aug;26(8):3723-3736. doi: 10.1038/s41380-019-0635-6. Epub 2020 Jan 3. Mol Psychiatry. 2021. PMID: 31900430 Free PMC article.
-
Functional Reorganization of Local Circuit Connectivity in Superficial Spinal Dorsal Horn with Neuropathic Pain States.eNeuro. 2019 Oct 10;6(5):ENEURO.0272-19.2019. doi: 10.1523/ENEURO.0272-19.2019. Print 2019 Sep/Oct. eNeuro. 2019. PMID: 31533959 Free PMC article.
-
Microglia Elimination Increases Neural Circuit Connectivity and Activity in Adult Mouse Cortex.J Neurosci. 2021 Feb 10;41(6):1274-1287. doi: 10.1523/JNEUROSCI.2140-20.2020. Epub 2020 Dec 30. J Neurosci. 2021. PMID: 33380470 Free PMC article.
-
Adaptive changes in the visual cortex after photoreceptor degeneration in retinitis pigmentosa.Histol Histopathol. 2025 Aug;40(8):1163-1172. doi: 10.14670/HH-18-891. Epub 2025 Feb 21. Histol Histopathol. 2025. PMID: 40047098 Review.
-
Theory of the Multiregional Neocortex: Large-Scale Neural Dynamics and Distributed Cognition.Annu Rev Neurosci. 2022 Jul 8;45:533-560. doi: 10.1146/annurev-neuro-110920-035434. Annu Rev Neurosci. 2022. PMID: 35803587 Free PMC article. Review.
References
-
- Anderson JC, Douglas RJ, Martin KA & Nelson JC (1994). Synaptic output of physiologically identified spiny stellate neurons in cat visual cortex. J Comp Neurol 341, 16–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

