Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents
- PMID: 26844979
- PMCID: PMC10329868
- DOI: 10.1002/14651858.CD009996.pub2
Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents
Abstract
Background: Attention deficit hyperactivity disorder (ADHD) is one of the most common psychiatric conditions affecting children and adolescents. Amphetamines are among the most commonly prescribed medications to manage ADHD. There are three main classes of amphetamines: dexamphetamine, lisdexamphetamine and mixed amphetamine salts, which can be further broken down into short- and long-acting formulations. A systematic review assessing their efficacy and safety in this population has never been conducted.
Objectives: To assess the efficacy and safety of amphetamines for ADHD in children and adolescents.
Search methods: In August 2015 we searched CENTRAL, Ovid MEDLINE, Embase, PsycINFO, ProQuest Dissertation and Theses, and the Networked Digital Library of Theses and Dissertations. We also searched ClinicalTrials.gov, and checked the reference lists of relevant studies and reviews identified by the searches. No language or date restrictions were applied.
Selection criteria: Parallel-group and cross-over randomized controlled trials (RCTs) comparing amphetamine derivatives against placebo in a pediatric population (< 18 years) with ADHD.
Data collection and analysis: Two authors independently extracted data on participants, settings, interventions, methodology, and outcomes for each included study. For continuous outcomes, we calculated the standardized mean difference (SMD) and for dichotomous outcomes we calculated the risk ratio (RR). Where possible, we conducted meta-analyses using a random-effects model. We also performed a meta-analysis of the most commonly reported adverse events in the primary studies.
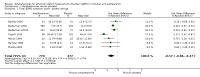
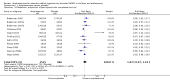
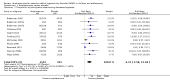
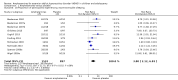
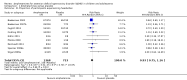
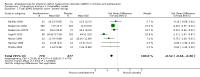
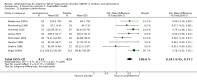
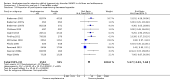
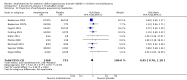
Main results: We included 23 trials (8 parallel-group and 15 cross-over trials), with 2675 children aged three years to 17 years. All studies compared amphetamines to placebo. Study durations ranged from 14 days to 365 days, with the majority lasting less than six months. Most studies were conducted in the United States; three studies were conducted across Europe. We judged 11 included studies to be at a high risk of bias due to insufficient blinding methods, failing to account for dropouts and exclusions from the analysis, and failing to report on all outcomes defined a priori. We judged the remaining 12 studies to be at unclear risk of bias due to inadequate reporting.Amphetamines improved total ADHD core symptom severity according to parent ratings (SMD -0.57; 95% confidence interval (CI) -0.86 to -0.27; 7 studies; 1247 children/adolescents; very low quality evidence), teacher ratings (SMD -0.55; 95% CI -0.83 to -0.27; 5 studies; 745 children/adolescents; low quality evidence), and clinician ratings (SMD -0.84; 95% CI -1.32 to -0.36; 3 studies; 813 children/adolescents; very low quality evidence). In addition, the proportion of responders as rated by the Clinical Global Impression - Improvement (CGI-I) scale was higher when children were taking amphetamines (RR 3.36; 95% CI 2.48 to 4.55; 9 studies; 2207 children/adolescents; very low quality evidence).The most commonly reported adverse events included decreased appetite, insomnia/trouble sleeping, abdominal pain, nausea/vomiting, headaches, and anxiety. Amphetamines were associated with a higher proportion of participants experiencing decreased appetite (RR 6.31; 95% CI 2.58 to 15.46; 11 studies; 2467 children/adolescents), insomnia (RR 3.80; 95% CI 2.12 to 6.83; 10 studies; 2429 children/adolescents), and abdominal pain (RR 1.44; 95% CI 1.03 to 2.00; 10 studies; 2155 children/adolescents). In addition, the proportion of children who experienced at least one adverse event was higher in the amphetamine group (RR 1.30; 95% CI 1.18 to 1.44; 6 studies; 1742 children/adolescents; low quality evidence).We performed subgroup analyses for amphetamine preparation (dexamphetamine, lisdexamphetamine, mixed amphetamine salts), amphetamine release formulation (long acting versus short acting), and funding source (industry versus non industry). Between-group differences were observed for proportion of participants experiencing decreased appetite in both the amphetamine preparation (P < 0.00001) and amphetamine release formulation (P value = 0.008) subgroups, as well as for retention in the amphetamine release formulation subgroup (P value = 0.03).
Authors' conclusions: Most of the included studies were at high risk of bias and the overall quality of the evidence ranged from low to very low on most outcomes. Although amphetamines seem efficacious at reducing the core symptoms of ADHD in the short term, they were associated with a number of adverse events. This review found no evidence that supports any one amphetamine derivative over another, and does not reveal any differences between long-acting and short-acting amphetamine preparations. Future trials should be longer in duration (i.e. more than 12 months), include more psychosocial outcomes (e.g. quality of life and parent stress), and be transparently reported.
Conflict of interest statement
Salima Punja ‐ none known. Larissa Shamseer ‐ none known. Lisa Hartling ‐ none known. Liana Urichuk ‐ received salary support from the Addiction & Mental Health Program of Alberta Health Services ‐ Edmonton Zone during the course of this review. Dr Urichuk also received a grant for a research project entitled "Neurofeedback for children with Attention Deficit Hyperactivity Disorder" from the Sick Kids Hospital Foundation and John and Lotte Hecht Memorial Foundation. Ben Vandermeer ‐ none known. Catherine Jane Nikles ‐ published a paper in the area of amphetamines for ADHD (Nikles 2006). The study was excluded due to the nature of the design. Dr Nikles was not involved in assessing the eligibility of this study, which was performed by two independent authors. Sunita Vohra ‐ is the recipient of an Alberta Innovates ‐ Health Scholar salary award. Dr Vohra's institution receives funds from Alberta Innovates ‐ Health Solutions and protects 75% of her time for research. Other funders include Health Canada, Canadian Institutes of Health Research, Women & Children’s Health Research Institute and National Health, Medical Research Council (Australia) and SERIN‐ETD Acupuncture Research Fund. SERIN is a commercial entity who provided acupuncture needles for a trial. The population is not pediatric ADHD. None of these companies pose a conflict of interest as regards this review.
Figures



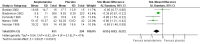
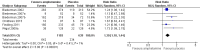



























































Update of
References
References to studies included in this review
Barkley 2000 {published data only}
-
- Barkley RA, Connor DF, Kwasnik D. Challenges to determining adolescent medication response in an outpatient clinical setting: comparing Adderall and methylphenidate for ADHD. Journal of Attention Disorders 2000;4(2):102‐13. [DOI: 10.1177/108705470000400204] - DOI
Biederman 2002 {published data only}
-
- Biederman J, Lopez FA, Boellner SW, Chandler MC. A randomized, double‐blind, placebo‐controlled, parallel‐group study of SLI381 (Adderall XR) in children with attention‐deficit/hyperactivity disorder. Pediatrics 2002;110(2):258‐66. - PubMed
Biederman 2007a {published data only}
-
- Biederman J, Boellner SW, Childress A, Lopez FA, Krishnan S, Zhang Y. Lisdexamfetamine dimesylate and mixed amphetamine salts extended‐release in children with ADHD: a double‐blind, placebo‐controlled, crossover analogue classroom study. Biological Psychiatry 2007;62(9):970‐6. [PUBMED: 17631866] - PubMed
Biederman 2007b {published data only (unpublished sought but not used)}
-
- Biederman J, Krishnan S, Zhang Y, McGough JJ, Findling RL. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP‐104) in children with attention‐deficit/hyperactivity disorder: a phase III, multicenter, randomized, double‐blind, forced‐dose, parallel‐group study. Clinical Therapeutics 2007;29(3):450‐63. [PUBMED: 17577466] - PubMed
Borcherding 1990 {published data only (unpublished sought but not used)}
-
- Borcherding BG, Keysor CS, Rapoport JL, Elia J, Amass J. Motor/vocal tics and compulsive behaviors on stimulant drugs: is there a common vulnerability?. Psychiatry Research 1990;33(1):83‐94. [PUBMED: 2217661] - PubMed
-
- Elia J, Borcherding BG, Rapoport JL, Keysor CS. Methylphenidate and dextroamphetamine treatments of hyperactivity: are there true nonresponders?. Psychiatry Research 1991;36(2):141‐55. [PUBMED: 2017529] - PubMed
Childress 2015 {published data only}
-
- Childress AC, Brams M, Culter AJ, Kollins SH, Northcutt J, Padilla A, et al. The efficacy and safety of evekeo, racemic amphetamine sulfate, for treatment of attention‐deficit/hyperactivity disorder symptoms: a multicenter, dose‐optimized, double‐blind, randomized, placebo‐controlled crossover laboratory classroom study. Journal of Child and Adolescent Psychopharmacology 2015;25(5):402‐14. [DOI: 10.1089/cap.2014.0176] - DOI - PMC - PubMed
Coghill 2013 {published data only}
-
- Banachewski T, Soutullo C, Lecendreux M, Johnson M, Zuddas A, Hodgkins P, et al. Health‐related quality of life and functional outcomes from a randomized, controlled study of lisdexamfetamine dimesylate in children and adolescents with attention deficit hyperactivity disorder. CNS Drugs 2013;27(10):829‐40. [DOI: 10.1007/s40263-013-0095-5] - DOI - PMC - PubMed
-
- Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology 2013;23(10):1208‐18. [DOI: 10.1016/j.euroneuro.2012.11.012] - DOI - PubMed
-
- Coghill DR, Banaschewski T, Lecendreux M, Zuddas A, Dittmann RW, Otero IH, et al. Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention‐deficit/hyperactivity disorder: results from a randomized, controlled trial. European Child and Adolescent Psychiatry 2014;23(2):61‐8. [PUBMED: 23708466] - PMC - PubMed
Donnelly 1989 {published data only (unpublished sought but not used)}
-
- Donnelly M, Rapoport JL, Ismond DR. Fenfluramine treatment of childhood attention deficit disorder with hyperactivity: a preliminary report. Psychopharmacology Bulletin 1986;22(1):152‐4. - PubMed
Findling 2011 {published data only}
-
- Findling RL, Childress AC, Cutler AJ, Gasior M, Hamdani M, Ferreira‐Cornwell MC, et al. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(4):395‐405. [PUBMED: 21421179] - PubMed
Giblin 2011 {published data only (unpublished sought but not used)}
Gillberg 1997 {published data only}
-
- Gillberg C, Melander H, Knorring AL, Janols LO, Thernlund G, Hagglof B, et al. Long‐term stimulant treatment of children with attention‐deficit hyperactivity disorder symptoms: a randomized, double‐blind, placebo‐controlled trial. Archives of General Psychiatry 1997;54(9):857‐64. [PUBMED: 9294377] - PubMed
James 2001 {published data only}
-
- James RS, Sharp WS, Bastain TM, Lee PP, Walter JM, Czarnolewski M, et al. Double‐blind, placebo‐controlled study of single‐dose amphetamine formulations in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(11):1268‐76. [PUBMED: 11699800] - PubMed
Manos 1999 {published data only}
-
- Manos MJ, Short EJ, Findling RL. Differential effectiveness of methylphenidate and Adderall in school‐age youths with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(7):813‐9. [PUBMED: 10405498] - PubMed
McCracken 2003 {published data only}
-
- McCracken JT, Biederman J, Greenhill LL, Swanson JM, McGough JJ, Spencer TJ, et al. Analog classroom assessment of a once‐daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(6):673‐83. [PUBMED: 12921475] - PubMed
Nemzer 1986 {published data only}
-
- Nemzer ED, Arnold E, Votolato NA, McConnell H. Amino acid supplementation as therapy for attention deficit disorder. Journal of the American Academy of Child Psychiatry 1986;25(4):509‐13. - PubMed
Pliszka 2000 {published data only}
-
- Pliszka SR, Browne RG, Olvera RL, Wynne SK. A double‐blind, placebo‐controlled study of Adderall and methylphenidate in the treatment of attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(5):619‐26. [DOI: 10.1097/00004583-200005000-00016] - DOI - PubMed
Ramtvedt 2013 {published data only}
-
- Ramtvedt BE, Aabech HS, Sundet K. Minimizing adverse events while maintaining clinical improvement in a pediatric attention‐deficit/hyperactivity disorder crossover trial with dextroamphetamine and methylphenidate. Journal of Child and Adolescent Psychopharmacology 2014;24(3):130‐9. [DOI: 10.1089/cap.2013.0114] - DOI - PMC - PubMed
Sharp 1999 {published data only (unpublished sought but not used)}
-
- Sharp WS, Walter JM, Marsh WL, Ritchie GF, Hamburger SD, Castellanos FX. ADHD in girls: clinical comparability of a research sample. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(1):40‐7. [PUBMED: 9893415] - PubMed
Shekim 1986 {published data only}
Short 2004 {published data only}
Spencer 2006a {published data only}
-
- Spencer TJ, Wilens TE, Biederman J, Weisler RH, Read SC, Pratt R. Efficacy and safety of mixed amphetamine salts extended release (Adderall XR) in the management of attention‐deficit/hyperactivity disorder in adolescent patients: a 4‐week, randomized, double‐blind, placebo‐controlled, parallel‐group study. Clinical Therapeutics 2006;28(2):266‐79. [DOI: 10.1016/j.clinthera.2006.02.011] - DOI - PubMed
Swanson 1998a {published data only (unpublished sought but not used)}
-
- Swanson JM, Wigal S, Greenhill LL, Browne R, Waslik B, Lerner M, et al. Analog classroom assessment of Adderall (R) in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37(5):519‐26. - PubMed
Wigal 2009a {published data only}
-
- Wigal SB, Kollins SH, Childress AC, Squires L, for the 311 Study Group. A 13‐hour laboratory school study of lisdexamfetamine dimesylate in school‐aged children with attention‐deficit/hyperactivity disorder. Child and Adolescent Psychiatry and Mental Health 2009;3(1):17. [DOI: 10.1186/1753-2000-3-17] - DOI - PMC - PubMed
References to studies excluded from this review
Akhondzadeh 2003 {published data only}
-
- Akhondzadeh S, Tavakolian R, Davari‐Ashtiani R, Arabgol F, Amini H. Selegiline in the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2003;27(5):841‐5. [DOI: 10.1016/S0278-5846(03)00117-9] - DOI - PubMed
Alexandris 1968 {published data only}
Arnold 2011 {published data only}
-
- Arnold LE, Disilvestro RA, Bozzolo D, Bozzolo H, Crowl L, Fernandez S, et al. Zinc for attention‐deficit/hyperactivity disorder: placebo‐controlled double‐blind pilot trial alone and combined with amphetamine. Journal of Child and Adolescent Psychopharmacology 2011;21(1):1‐19. [PUBMED: 21309695] - PMC - PubMed
Biederman 2006 {published data only}
-
- Biederman J, Wigal SB, Spencer TJ, McGough JJ, Mays DA. A post hoc subgroup analysis of an 18‐day randomized controlled trial comparing the tolerability and efficacy of mixed amphetamine salts extended release and atomoxetine in school‐age girls with attention‐deficit/hyperactivity disorder. Clinical Therapeutics 2006;28(2):280‐93. [DOI: 10.1016/j.clinthera.2006.02.008] - DOI - PubMed
Biederman 2008 {published data only}
-
- Biederman J, Seidman LJ, Petty CR, Fried R, Doyle AE, Cohen DR, et al. Effects of stimulant medication on neuropsychological functioning in young adults with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2008;69(7):1150‐6. - PubMed
Boellner 2010 {published data only}
-
- Boellner SW, Stark JG, Krishnan S, Zhang Y. Pharmacokinetics of lisdexamfetamine dimesylate and its active metabolite, d‐amphetamine, with increasing oral doses of lisdexamfetamine dimesylate in children with attention‐deficit/hyperactivity disorder: a single‐dose, randomized, open‐label, crossover study. Clinical Therapeutics 2010;32(2):252‐64. [PUBMED: 20206783] - PubMed
Brown 2010 {published data only}
-
- Brown TE, Landgraf JM. Improvements in executive function correlate with enhanced performance and functioning and health‐related quality of life: evidence from 2 large, double‐blind, randomized, placebo‐controlled trials in ADHD. Postgraduate Medicine 2010;122(5):42‐51. [PUBMED: 20861587] - PubMed
Denhoff 1971 {published data only}
-
- Denhoff E, Davids A, Hawkins R. Effects of dextroamphetamine on hyperkinetic children: a controlled double blind study. Journal of Learning Disabilities 1971;4(9):491‐8. [DOI: 10.1177/002221947100400906] - DOI
Donner 2007 {published data only}
Efron 1997 {published data only}
-
- Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double‐blind, crossover trial. Pediatrics 1997;100(4):662‐6. [PUBMED: 9310521] - PubMed
Findling 2009 {published data only}
-
- Findling RL, Ginsberg LD, Jain R, Gao J. Effectiveness, safety, and tolerability of lisdexamfetamine dimesylate in children with attention‐deficit/hyperactivity disorder: an open‐label, dose‐optimization study. Journal of Child and Adolescent Psychopharmacology 2009;19(6):649‐62. [PUBMED: 20035583] - PubMed
Greenhill 2003 {published data only}
-
- Greenhill LL, Swanson JM, Steinhoff K, Fried J, Posner K, Lerner M, et al. A pharmacokinetic/pharmacodynamic study comparing a single morning dose of Adderall to twice‐daily dosing in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(10):1234‐41. [PUBMED: 14560174] - PubMed
Kamien 1998 {published data only}
-
- Kamien M. The use of an N‐of‐1 randomised clinical trial in resolving therapeutic doubt. The case of a patient with an 'attention disorder'. Australian Family Physician 1998;27 Suppl 2:S103‐5. - PubMed
Lopez 2008 {published data only}
McGough 2005 {published data only}
-
- McGough JJ, Biederman J, Wigal SB, Lopez FA, McCracken JT, Spencer T, et al. Long‐term tolerability and effectiveness of once‐daily mixed amphetamine salts (Adderall XR) in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(6):530‐8. [DOI: 10.1097/01.chi.0000157550.94702.a2] - DOI - PubMed
Najib 2009 {published data only}
Nikles 2006 {published data only}
-
- Nikles CJ, Mitchell GK, Mar CB, Clavarino A, McNairn N. An n‐of‐1 trial service in clinical practice: testing the effectiveness of stimulants for attention‐deficit/hyperactivity disorder. Pediatrics 2006;117(6):2040‐6. - PubMed
Quintana 2007 {published data only}
-
- Quintana H, Cherlin EA, Duesenberg DA, Bangs ME, Ramsey JL, Feldman PD, et al. Transition from methylphenidate or amphetamine to atomoxetine in children and adolescents with attention‐deficit/hyperactivity disorder ‐ a preliminary tolerability and efficacy study. Clinical Therapeutics 2007;29(6):1168‐77. [DOI: 10.1016/j.clinthera.2007.06.017] - DOI - PubMed
Scheffer 2005 {published data only}
-
- Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo‐controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. American Journal of Psychiatry 2005;162(1):58‐64. [PUBMED: 15625202] - PubMed
Sleator 1974 {published data only}
Spencer 2005 {published data only}
-
- Spencer TJ, Biederman J, Wilens TE. Efficacy and tolerability of long‐term, open‐label, mixed amphetamine salts extended release in adolescents with ADHD. CNS Spectrums 2005;10 Suppl 15:14‐21. [DOI: 10.1017/S1092852900014103] - DOI
Spencer 2006b {published data only}
-
- Spencer TJ, Abikoff HB, Connor DF, Biederman J, Pliszka SR, Boellner S, et al. Efficacy and safety of mixed amphetamine salts extended release (Adderall XR) in the management of oppositional defiant disorder with or without comorbid attention‐deficit/hyperactivity disorder in school‐aged children and adolescents: a 4‐week, multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled, forced‐dose‐escalation study. Clinical Therapeutics 2006;28(3):402‐18. [DOI: 10.1016/j.clinthera.2006.03.006] - DOI - PubMed
Turgay 2010 {published data only}
-
- Turgay A, Ginsberg L, Sarkis E, Jain R, Adeyi B, Gao J, et al. Executive function deficits in children with attention‐deficit/hyperactivity disorder and improvement with lisdexamfetamine dimesylate in an open‐label study. Journal of Child and Adolescent Psychopharmacology 2010;20(6):503‐11. [DOI: 10.1089/cap.2009.0110] - DOI - PMC - PubMed
Wigal 2009b {published data only}
-
- Wigal SB, Kollins SH, Childress AC, Squires L, for the 311 Study Group. A 13‐hour laboratory school study of lisdexamfetamine dimesylate in school‐aged children with attention‐deficit/hyperactivity disorder. Child and Adolescent Psychiatry and Mental Health 2009;3(1):17. [DOI: 10.1186/1753-2000-3-17] - DOI - PMC - PubMed
Wigal 2010a {published data only}
-
- Wigal SB, Kollins SH, Childress AC, Adeyi B. Efficacy and tolerability of lisdexamfetamine dimesylate in children with attention‐deficit/hyperactivity disorder: sex and age effects and effect size across the day. Child and Adolescent Psychiatry and Mental Health 2010;4:32. [DOI: 10.1186/1753-2000-4-32] - DOI - PMC - PubMed
Wigal 2010b {published data only}
-
- Wigal T, Brams M, Gasior M, Gao J, Squires L, Giblin J, et al. Randomized, double‐blind, placebo‐controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention‐deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design. Behavioral and Brain Functions 2010;6:34. [DOI: 10.1186/1744-9081-6-34] - DOI - PMC - PubMed
References to studies awaiting assessment
Glos 1973 {published data only}
-
- Glos J. Amphetamines in the treatment of hyperactive and unstable children. Ceskoslovenska Pediatrie 1973;28(10):559‐60. - PubMed
Itil 1974 {published data only}
-
- Itil TM, Simeon J. Proceedings: computerized EEG in the prediction of outcome of drug treatment in hyperactive childhood behavior disorders. Psychopharmacology Bulletin 1974;10(4):36. [PUBMED: 4610619] - PubMed
References to ongoing studies
Fanton 2009 {published data only}
-
- Fanton J, Waslick B, Harvey E. The 49th Annual National Institute of Mental Health (NIMH) New Clinical Drug Evaluation Unit (NCDEU) meeting Hollywood, Florida, June 29‐July 2, 2009: posters most relevant to child and adolescent psychopharmacology. Journal of Child and Adolescent Psychopharmacology 2009; Vol. 19, issue 6:786. - PubMed
NCT01711021 {published data only}
-
- NCT01711021. A randomized, double‐blind, placebo‐controlled, crossover, laboratory classroom study to evaluate the safety and efficacy of d‐amphetamine transdermal drug delivery system (d‐ATS) compared to placebo in children and adolescents with ADHD. http://clinicaltrials.gov/ct2/show/record/NCT01711021 (accessed 11 February 2014).
Additional references
AHRQ 1999
-
- Agency for Health Care Policy and Research. Diagnosis of attention‐deficit/hyperactivity disorder. Summary: technical review: number 3. www.ahrq.gov/clinic/epcsums/adhdsutr.htm (accessed 1 May 2012).
Anderson 1999
-
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Natural Neuroscience 1999;2(11):1032‐7. - PubMed
APA 1987
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III‐R). 3rd Edition. Washington, DC: American Psychiatric Association, 1987.
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR). 4th Edition. Washington, DC: American Psychiatric Association, 2000.
Arnsten 2006
-
- Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology 2006;31(11):2376‐83. - PubMed
Bero 2013
Biederman 1993
-
- Biederman J, Faraone SV, Spencer T, Wilens T, Norman D, Lapey KA, et al. Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. American Journal of Psychiatry 1993;150(12):1792‐8. - PubMed
Buck 2002
-
- Buck ML. Amphetamines in the treatment of attention‐deficit/hyperactivity disorder. Pediatric Pharmacotherapy 2002;8(3):1‐3.
Castells 2011
Charach 2011
-
- Charach A, Dashti B, Carson P, Booker L, Lim CG, Lillie E, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at‐risk preschoolers; long‐term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Agency for Healthcare Research and Quality 2011. - PubMed
Cohen 1988
-
- Cohen J. Statistical Power Analysis in Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates Inc, 1988.
Conners 1990
-
- Conners CK. Conners’ Abbreviated Symptom Questionnaire: Parent Version, Teacher Version Manual. Toronto: Multi‐Health Systems, 1990.
Conners 1998a
-
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS‐R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):257‐68. - PubMed
Conners 1998b
-
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS‐R): factor structure, reliability and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):279‐91. - PubMed
Costello 1985
-
- Costello EJ, Edelbrock CS, Costello AJ. Validity of the NIMH Diagnostic Interview Schedule for Children: a comparison between psychiatric and pediatric referrals. Journal of Abnormal Child Psychology 1985;13(4):579‐95. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DuPaul 1998
-
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale—IV: Checklists, Norms, and Clinical Interpretation. New York: The Guilford Press, 1998:186‐201.
Edwards 1995
-
- Edwards L, Salant V, Howard VF, Brougher J, McLaughlin TF. Effectiveness of self‐management on attentional behavior and reading comprehension for children with attention deficit disorder. Child and Family Behavior Therapy 1995;17(2):1‐17. [DOI: 10.1300/J019v17n02_01] - DOI
Egger 1997
Follman 1992
GRADE Working Group 2004
Guy 1976
-
- Busner J, Targum SD. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health, 1976.
Hedges 1994
-
- Hedges LV. Statistical considerations. In: Cooper H, Hedges LV (editors). The Handbook of Research Synthesis. New York (NY): Russell Sage Foundation 1994.
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lundh 2012
Mayes 2009
Miller 1999
-
- Miller A, Lee S, Raina P, Klassen A, Zupancic J, Olsen L. A review of therapies for attention‐deficit/hyperactivity disorder. www.cadth.ca/en/products/health‐technology‐assessment/publication/20 (accessed 11 February 2014).
Moher 2003
-
- Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomized trials published in languages other than English in systematic reviews. Health Technology Assessment 2003;7(41):1‐90. - PubMed
Moore 2011
-
- Moore EA. Ritalin, Adderall and other treatments for ADHD. The Amphetamine Debate: The Use of Adderall, Ritalin and Related Drugs for Behavior Modification, Neuroenhancement, and Anti‐Aging Purposes. Jefferson, North Carolina: McFarland and Company, Inc, 2011:80‐107.
NIH 2000
-
- National Institute of Health. National Institutes of Health consensus development conference statement: diagnosis and treatment of attention‐deficit/hyperactivity disorder (ADHD). Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(2):182‐97. - PubMed
NIMH 2009
-
- National Institute for Mental Health. Attention Deficit Hyperactivity Disorder (ADHD). www.nimh.nih.gov/health/publications/attention‐deficit‐hyperactivity‐dis... (accessed 1 May 2012).
Polanczyk 2007
-
- Polanczyk G, Silva de Lima M, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metagression analysis. American Journal of Psychiatry 2007;164(6):942‐8. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salum 2012
-
- Salum GA, Patrick DL, Isolan LR, Manfro GG, Fleck MP. Youth Quality of Life Instrument‐Research version (YQOL‐R): psychometric properties in a community sample [Youth Quality of Life Instrument‐Research version (YQOL‐R): propriedades psicométricas em uma amostra comunitária]. Journal of Pediatrics 2012;88(5):443‐8. [PUBMED: 22850664] - PubMed
Schulz 2010
Sterne 2013
Swanson 1998b
-
- Swanson J, Wigal S, Greenhill L, Browne R, Waslick B, Lerner M, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacoly Bulletin 1998;34(1):55‐60. [PUBMED: 9564199] - PubMed
Swanson 2007
-
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. Etiologic subtypes of attention‐deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychology Reviews 2007;17(1):39‐59. [PUBMED: 17318414] - PubMed
The Medical Letter 2007
-
- The Medical Letter Online. Lisdexamfetamine dimesylate (Vyvanase) for ADHD. The Medical Letter on Drugs and Therapeutics 2007;49(1265):58‐9. - PubMed
Varni 1998
-
- Varni JW, Katz ER, Seid M, Quiggins DJ, Friedman‐Bender A. The Pediatric Quality of Life Inventory‐32 (PedsQL‐32): reliability and validity. Cancer 1998;82(6):1184‐96. [PUBMED: 9506367] - PubMed
Wechsler 1991
-
- Wechsler D. Wechsler Intelligence Scale for Children. San Antonio, TX: The Psychological Corporation, 1991.
Wigal 1999
-
- Wigal T, Swanson JM, Regino R, Lerner MA, Soliman I, Steinoff K, et al. Stimulant medications for the treatment of ADHD: efficacy and limitations. Mental Retardation and Development Disabilities Research Reviews 1999;5(3):215‐24.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

