Evidence of Alternative Cystatin C Signal Sequence Cleavage Which Is Influenced by the A25T Polymorphism
- PMID: 26845025
- PMCID: PMC4741414
- DOI: 10.1371/journal.pone.0147684
Evidence of Alternative Cystatin C Signal Sequence Cleavage Which Is Influenced by the A25T Polymorphism
Abstract
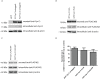
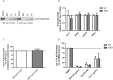
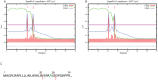
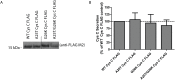
Cystatin C (Cys C) is a small, potent, cysteine protease inhibitor. An Ala25Thr (A25T) polymorphism in Cys C has been associated with both macular degeneration and late-onset Alzheimer's disease. Previously, studies have suggested that this polymorphism may compromise the secretion of Cys C. Interestingly, we found that untagged A25T, A25T tagged C-terminally with FLAG, or A25T FLAG followed by green fluorescent protein (GFP), were all secreted as efficiently from immortalized human cells as their wild-type (WT) counterparts (e.g., 112%, 100%, and 88% of WT levels from HEK-293T cells, respectively). Supporting these observations, WT and A25T Cys C variants also showed similar intracellular steady state levels. Furthermore, A25T Cys C did not activate the unfolded protein response and followed the same canonical endoplasmic reticulum (ER)-Golgi trafficking pathway as WT Cys C. WT Cys C has been shown to undergo signal sequence cleavage between residues Gly26 and Ser27. While the A25T polymorphism did not affect Cys C secretion, we hypothesized that it may alter where the Cys C signal sequence is preferentially cleaved. Under normal conditions, WT and A25T Cys C have the same signal sequence cleavage site after Gly26 (referred to as 'site 2' cleavage). However, in particular circumstances when the residues around site 2 are modified (such as by the presence of an N-terminal FLAG tag immediately after Gly26, or by a Gly26Lys (G26K) mutation), A25T has a significantly higher likelihood than WT Cys C of alternative signal sequence cleavage after Ala20 ('site 1') or even earlier in the Cys C sequence. Overall, our results indicate that the A25T polymorphism does not cause a significant reduction in Cys C secretion, but instead predisposes the protein to be cleaved at an alternative signal sequence cleavage site if site 2 is hindered. Additional N-terminal amino acids resulting from alternative signal sequence cleavage may, in turn, affect the protease inhibition function of Cys C.
Conflict of interest statement
Figures

Similar articles
-
Cystatins--Extra- and intracellular cysteine protease inhibitors: High-level secretion and uptake of cystatin C in human neuroblastoma cells.Biochimie. 2010 Nov;92(11):1625-34. doi: 10.1016/j.biochi.2010.08.011. Epub 2010 Aug 25. Biochimie. 2010. PMID: 20800088
-
Unexpected intracellular localization of the AMD-associated cystatin C variant.Traffic. 2004 Nov;5(11):884-95. doi: 10.1111/j.1600-0854.2004.00230.x. Traffic. 2004. PMID: 15479453
-
Cystatin C in macular and neuronal degenerations: implications for mechanism(s) of age-related macular degeneration.Vision Res. 2010 Mar 31;50(7):737-42. doi: 10.1016/j.visres.2009.10.022. Epub 2009 Nov 14. Vision Res. 2010. PMID: 19917302
-
Amyloid properties of the leader peptide of variant B cystatin C: implications for Alzheimer and macular degeneration.FEBS Lett. 2016 Mar;590(5):644-54. doi: 10.1002/1873-3468.12093. Epub 2016 Feb 26. FEBS Lett. 2016. PMID: 26865059
-
Cystatin C attenuates insulin signaling transduction by promoting endoplasmic reticulum stress in hepatocytes.FEBS Lett. 2015 Dec 21;589(24 Pt B):3938-44. doi: 10.1016/j.febslet.2015.11.029. Epub 2015 Nov 22. FEBS Lett. 2015. PMID: 26592151
Cited by
-
Clinically-identified C-terminal mutations in fibulin-3 are prone to misfolding and destabilization.Sci Rep. 2021 Feb 4;11(1):2998. doi: 10.1038/s41598-020-79570-x. Sci Rep. 2021. PMID: 33542268 Free PMC article.
-
Select azo compounds post-translationally modulate HTRA1 abundance and activity potentially through interactions at the trimer interface.bioRxiv [Preprint]. 2025 May 16:2025.05.13.651909. doi: 10.1101/2025.05.13.651909. bioRxiv. 2025. Update in: ACS Chem Biol. 2025 Aug 15;20(8):1849-1862. doi: 10.1021/acschembio.4c00818. PMID: 40463243 Free PMC article. Updated. Preprint.
-
Structural and functional analysis of cystatin E reveals enzymologically relevant dimer and amyloid fibril states.J Biol Chem. 2018 Aug 24;293(34):13151-13165. doi: 10.1074/jbc.RA118.002154. Epub 2018 Jul 2. J Biol Chem. 2018. PMID: 29967063 Free PMC article.
-
Cystatins: unravelling the biological implications for neuroprotection.Arch Med Sci. 2023 Sep 19;20(1):157-166. doi: 10.5114/aoms/171706. eCollection 2024. Arch Med Sci. 2023. PMID: 38414464 Free PMC article.
-
GSK3 inhibition reduces ECM production and prevents age-related macular degeneration-like pathology.bioRxiv [Preprint]. 2023 Dec 15:2023.12.14.571757. doi: 10.1101/2023.12.14.571757. bioRxiv. 2023. Update in: JCI Insight. 2024 Aug 8;9(15):e178050. doi: 10.1172/jci.insight.178050. PMID: 38168310 Free PMC article. Updated. Preprint.
References
-
- Paraoan L, Grierson I, Maden BE. Analysis of expressed sequence tags of retinal pigment epithelium: cystatin C is an abundant transcript. The international journal of biochemistry & cell biology. 2000;32(4):417–26. . - PubMed
-
- Barrett AJ, Davies ME, Grubb A. The place of human gamma-trace (cystatin C) amongst the cysteine proteinase inhibitors. Biochemical and biophysical research communications. 1984;120(2):631–6. . - PubMed
-
- Jones A, Kumar S, Zhang N, Tong Z, Yang JH, Watt C, et al. Increased expression of multifunctional serine protease, HTRA1, in retinal pigment epithelium induces polypoidal choroidal vasculopathy in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(35):14578–83. 10.1073/pnas.1102853108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

