Sequestering survivin to functionalized nanoparticles: a strategy to enhance apoptosis in cancer cells
- PMID: 26845086
- PMCID: PMC4803599
- DOI: 10.1039/c5bm00580a
Sequestering survivin to functionalized nanoparticles: a strategy to enhance apoptosis in cancer cells
Abstract
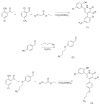
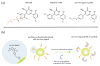
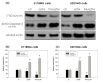
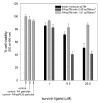
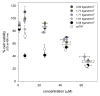
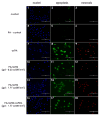
Survivin belongs to the family of inhibitor of apoptosis proteins (IAP) and is present in most cancers while being below detection limits in most terminally differentiated adult tissues, making it an attractive protein to target for diagnostic and, potentially, therapeutic roles. Sub-100 nm poly(propargyl acrylate) (PA) particles were surface modified through the copper-catalyzed azide/alkyne cycloaddition of an azide-terminated survivin ligand derivative (azTM) originally proposed by Abbott Laboratories and speculated to bind directly to survivin (protein) at its dimer interface. Using affinity pull-down studies, it was determined that the PA/azTM nanoparticles selectively bind survivin and the particles can enhance apoptotic cell death in glioblastoma cell lines and other survivin over-expressing cell lines such as A549 and MCF7 relative to cells incubated with the original Abbott-derived small molecule inhibitor.
Figures
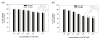



Similar articles
-
Chemically synthesized human survivin does not inhibit caspase-3.Protein Sci. 2008 Sep;17(9):1624-9. doi: 10.1110/ps.036145.108. Epub 2008 Jun 6. Protein Sci. 2008. PMID: 18539906 Free PMC article.
-
An IAP in action: the multiple roles of survivin in differentiation, immunity and malignancy.Cell Cycle. 2004 Sep;3(9):1121-3. Epub 2004 Sep 15. Cell Cycle. 2004. PMID: 15326382 Review.
-
Cartilage oligomeric matrix protein protects cells against death by elevating members of the IAP family of survival proteins.J Biol Chem. 2008 Jan 4;283(1):648-659. doi: 10.1074/jbc.M704035200. Epub 2007 Nov 8. J Biol Chem. 2008. PMID: 17993464
-
[Survivin--a new tumor-specific anti-apoptosis factor].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2001 Oct;23(5):532-4. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2001. PMID: 12905879 Review. Chinese.
-
Survivin: an inhibitor of apoptosis in pediatric cancer.Pediatr Blood Cancer. 2006 Jul;47(1):4-13. doi: 10.1002/pbc.20805. Pediatr Blood Cancer. 2006. PMID: 16534789 Review.
Cited by
-
Survivin Interference and SurVaxM as an Adjunct Therapy for Glioblastoma Multiforme.Cells. 2025 May 21;14(10):755. doi: 10.3390/cells14100755. Cells. 2025. PMID: 40422257 Free PMC article. Review.
References
-
- Altieri DC. Nature Reviews Cancer. 2008;8:61–70. - PubMed
-
- Li FZ, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Nature. 1998;396:580–584. - PubMed
-
- Ambrosini G, Adida C, Altieri DC. Nature Medicine. 1997;3:917–921. - PubMed
-
- Altieri DC. Nature Reviews Cancer. 2003;3:46–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

