The role of iron in the management of chemotherapy-induced anemia in cancer patients receiving erythropoiesis-stimulating agents
- PMID: 26845108
- PMCID: PMC8765740
- DOI: 10.1002/14651858.CD009624.pub2
The role of iron in the management of chemotherapy-induced anemia in cancer patients receiving erythropoiesis-stimulating agents
Abstract
Background: Erythropoiesis-stimulating agents (ESAs) are commonly used to treat chemotherapy-induced anemia (CIA). However, about half of patients do not benefit.
Objectives: To evaluate the benefits and harms related to the use of iron as a supplement to ESA and iron alone compared with ESA alone in the management of CIA.
Search methods: We searched for relevant trials from the Cochrane Central Register of Controlled Trials (CENTRAL) (issue 1 January 2016), MEDLINE (1950 to February 2016), and www.clinicaltrials.gov without using any language limits.
Selection criteria: All randomized controlled trials (RCTs) comparing 'iron plus ESA' or 'iron alone' versus 'ESA alone' in people with CIA were eligible for inclusion.
Data collection and analysis: We used standard methodological procedures expected by Cochrane.
Main results: We included eight RCTs (12 comparisons) comparing ESA plus iron versus ESA alone enrolling 2087 participants. We did not find any trial comparing iron alone versus ESAs alone in people with CIA. None of the included RCTs reported overall survival. There was a beneficial effect of iron supplementation to ESAs compared with ESAs alone on hematopoietic response (risk ratio (RR) 1.17, 95% confidence interval (CI) 1.09 to 1.26; P < 0.0001; 1712 participants; 11 comparisons; high-quality evidence). Assuming a baseline risk of 35% to 80% for hematopoietic response without iron supplementation, between seven and 16 patients should be treated to achieve hematopoietic response in one patient. In subgroup analyses, RCTs that used intravenous (IV) iron favored ESAs and iron (RR 1.20 (95% CI 1.10 to 1.31); P < 0.00001; 1321 participants; eight comparisons), whereas we found no evidence for a difference in hematopoietic response in RCTs using oral iron (RR 1.04 (95% CI 0.87 to 1.24); P = 0.68; 391 participants; three comparisons). There was no evidence for a difference between the subgroups of IV and oral iron (P = 0.16). There was no evidence for a difference between the subgroups of types of iron (P = 0.31) and types of ESAs (P = 0.16) for hematopoietic response.The iron supplementation to ESAs might be beneficial as fewer participants treated with iron supplementation required red blood cell (RBC) transfusions compared to the number of participants treated with ESAs alone (RR 0.74 (95% CI 0.60 to 0.92); P = 0.007; 1719 participants; 11 comparisons; moderate-quality evidence). Assuming a baseline risk of 7% to 40% for RBC transfusion without iron supplementation, between 10 and 57 patients should be treated to avoid RBC transfusion in one patient.We found no evidence for a difference in the median time to hematopoietic response with addition of iron to ESAs (hazard ratio (HR) 0.93 (95% CI 0.67 to 1.28); P = 0.65; 1042 participants; seven comparisons; low-quality evidence). In subgroup analyses, RCTs in which dextran (HR 0.95 (95% CI 0.36 to 2.52); P = 0.92; 340 participants; three comparisons), sucrose iron (HR 1.15 (95% CI 0.60 to 2.21); P = 0.67; 102 participants; one comparison) and sulfate iron (HR 1.24 (95% CI 0.99 to 1.56); P = 0.06; 55 participants; one comparison) were used showed no evidence for difference between iron supplementation versus ESAs alone compared with RCTs in which gluconate (HR 0.78 (95% CI 0.65 to 0.94); P = 0.01; 464 participants; two comparisons) was used for median time to hematopoietic response (P = 0.02). There was no evidence for a difference between the subgroups of route of iron administration (P = 0.13) and types of ESAs (P = 0.46) for median time to hematopoietic response.Our results indicated that there could be improvement in the hemoglobin (Hb) levels with addition of iron to ESAs (mean difference (MD) 0.48 (95% CI 0.10 to 0.86); P = 0.01; 827 participants; seven comparisons; low-quality evidence). In RCTs in which IV iron was used there was evidence for a difference (MD 0.84 (95% CI 0.21 to 1.46); P = 0.009; 436 participants; four comparisons) compared with oral iron (MD 0.07 (95% CI -0.19 to 0.34); P = 0.59; 391 participants; three comparisons) for mean change in Hb level (P = 0.03). RCTs in which dextran (MD 1.55 (95% CI 0.62 to 2.47); P = 0.001; 102 participants; two comparisons) was used showed evidence for a difference with iron supplementation versus ESAs alone compared with RCTs in which gluconate (MD 0.54 (95% CI -0.15 to 1.22); P = 0.12; 334 participants; two comparisons) and sulfate iron (MD 0.07 (95% CI -0.19 to 0.34); P = 0.59; 391 participants; three comparisons) were used for mean change in Hb level (P = 0.007). RCTs in which epoetin was used showed evidence for a difference with iron supplementation versus ESAs alone (MD 0.77 (95% CI 0.25 to 1.29); P = 0.004; 337 participants; five comparisons) compared with darbepoetin use (MD 0.10 (95% CI -0.13 to 0.33); P = 0.38; 490 participants; two comparisons) for mean change in Hb level (P = 0.02).We found no evidence for a difference in quality of life with addition of iron to ESAs (standardized mean difference 0.01 (95% CI -0.10 to 0.12); P = 0.88; 1124 participants; three RCTs; high-quality evidence).We found no evidence for a difference in risk of grade III-IV thromboembolic events (RR 0.95 (95% CI 0.54 to 1.65); P = 0.85; 783 participants; three RCTs; moderate-quality evidence). The incidence of treatment-related mortality (TRM) was 0% (997 participants; four comparisons; high-quality evidence).Other common adverse events included vomiting, asthenia, and leukopenia, and were similar in both arms.Overall the risk of bias across outcomes was high to low. Since the included RCTs had shorter follow-up duration (up to 20 weeks), the long-term effects of iron supplementation are unknown. Our main reasons for downgrading the quality of evidence were inconsistency across the included studies and imprecision of results.
Authors' conclusions: Our systematic review shows that addition of iron to ESAs offers superior hematopoietic response, reduces the risk of RBC transfusions, and improves Hb levels, and appears to be well tolerated. None of the included RCTs reported overall survival. We found no evidence for a difference in quality of life with iron supplementation.
Conflict of interest statement
Rahul Mhaksar: None
Hesborn Wao: None
Branko Miladinovic: None
Ambuj Kumar: None
Benjamin Djulbegovic: None
Figures
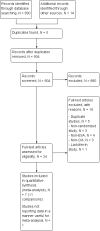
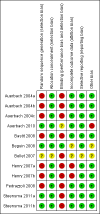
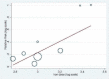































Update of
- doi: 10.1002/14651858.CD009624
Similar articles
-
Intravenous iron versus oral iron versus no iron with or without erythropoiesis- stimulating agents (ESA) for cancer patients with anaemia: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2022 Jun 20;6(6):CD012633. doi: 10.1002/14651858.CD012633.pub2. Cochrane Database Syst Rev. 2022. PMID: 35724934 Free PMC article.
-
Restrictive versus liberal red blood cell transfusion strategies for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support.Cochrane Database Syst Rev. 2017 Jan 27;1(1):CD011305. doi: 10.1002/14651858.CD011305.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2024 May 23;5:CD011305. doi: 10.1002/14651858.CD011305.pub3. PMID: 28128441 Free PMC article. Updated.
-
Pathogen-reduced platelets for the prevention of bleeding.Cochrane Database Syst Rev. 2017 Jul 30;7(7):CD009072. doi: 10.1002/14651858.CD009072.pub3. Cochrane Database Syst Rev. 2017. PMID: 28756627 Free PMC article.
-
A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment.Health Technol Assess. 2007 Apr;11(13):1-202, iii-iv. doi: 10.3310/hta11130. Health Technol Assess. 2007. PMID: 17408534
-
Interventions for infantile haemangiomas of the skin.Cochrane Database Syst Rev. 2018 Apr 18;4(4):CD006545. doi: 10.1002/14651858.CD006545.pub3. Cochrane Database Syst Rev. 2018. PMID: 29667726 Free PMC article.
Cited by
-
Effect of erythropoiesis-stimulating agents on breast cancer patients: a meta-analysis.Clin Exp Med. 2023 Sep;23(5):1501-1513. doi: 10.1007/s10238-022-00921-1. Epub 2022 Oct 31. Clin Exp Med. 2023. PMID: 36315312 Review.
-
Experimental Drugs for Chemotherapy- and Cancer-Related Anemia.J Exp Pharmacol. 2021 Jun 24;13:593-611. doi: 10.2147/JEP.S262349. eCollection 2021. J Exp Pharmacol. 2021. PMID: 34194245 Free PMC article. Review.
-
An evaluation of the recommendations for primary nutrition research addressing noncommunicable disease using the EPICOT+ framework: A cross-sectional descriptive meta-research study of Cochrane nutrition systematic reviews.Cochrane Evid Synth Methods. 2024 Mar 18;2(3):e12048. doi: 10.1002/cesm.12048. eCollection 2024 Mar. Cochrane Evid Synth Methods. 2024. PMID: 40475187 Free PMC article.
-
Anemia in patients receiving anticancer treatments: focus on novel therapeutic approaches.Front Oncol. 2024 Apr 2;14:1380358. doi: 10.3389/fonc.2024.1380358. eCollection 2024. Front Oncol. 2024. PMID: 38628673 Free PMC article. Review.
-
Peri-operative blood management of Jehovah's Witnesses undergoing cytoreductive surgery for advanced ovarian cancer.Blood Transfus. 2022 Mar;20(2):112-119. doi: 10.2450/2021.0416-20. Epub 2021 Feb 25. Blood Transfus. 2022. PMID: 35244533 Free PMC article.
References
References to studies included in this review
Auerbach 2004a {published data only}
-
- Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy‐related anemia: a multicenter, open‐label, randomized trial. Journal of Clinical Oncology 2004;22(7):1301‐7. - PubMed
Auerbach 2004b {published data only}
-
- Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy‐related anemia: a multicenter, open‐label, randomized trial. Journal of Clinical Oncology 2004;22(7):1301‐7. - PubMed
Auerbach 2004c {published data only}
-
- Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy‐related anemia: a multicenter, open‐label, randomized trial. Journal of Clinical Oncology 2004;22(7):1301‐7. - PubMed
Auerbach 2010 {published data only}
-
- Auerbach M, Silberstein PT, Webb RT, Averyanova S, Ciuleanu T, Shao J, et al. Darbepoetin alfa 300 or 500 μg once every 3 weeks with or without intravenous iron in patients with chemotherapy‐induced anemia. American Journal of Hematology 2010;85(9):655‐63. - PubMed
Bastit 2008 {published data only}
-
- Bastit L, Vandebroek A, Altintas S, Gaede B, Pinter T, Suto TS, et al. Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy‐induced anemia. Journal of Clinical Oncology 2008;26(10):1611‐8. - PubMed
Beguin 2008 {published data only}
-
- Beguin Y, Maertens J, Prijck B, Schots R, Frere P, Bonnet C, et al. Darbepoetin‐alfa and I.V. iron administration after autologous hematopoietic stem cell transplantation: A prospective randomized multicenter trial. American Society of hematology Annual Meeting and Exposition. 2008. - PubMed
Bellet 2007 {published data only}
-
- Bellet RE, Ghazal H, Flam M, Drelichman A, Gabrail N, Woytowitz D, et al. A phase III randomized controlled study comparing iron sucrose intravenously (IV) to no iron treatment of anemia in cancer patients undergoing chemotherapy and erythropoietin stimulating agent (ESA) therapy. Journal of Clinical Oncology, 2007 ASCO Annual Meetings Proceedings Part 1 2007;25(18S):9109.
Henry 2007a {published data only}
-
- Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 2007;12(2):231‐42. - PubMed
Henry 2007b {published data only}
-
- Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 2007;12(2):231‐42. - PubMed
Pedrazzoli 2008 {published data only}
-
- Pedrazzoli P, Farris A, Prete S, Gaizo F, Ferrari D, Bianchessi C, et al. Randomized trial of intravenous iron supplementation in patients with chemotherapy‐related anemia without iron deficiency treated with darbepoetin alpha. Journal of Clinical Oncology 2008;26(10):1619‐25. - PubMed
Steensma 2011a {published data only}
-
- Steensma DP, Sloan JA, Dakhil SR, Dalton R, Kahanic SP, Prager DJ, et al. Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy‐associated anemia. Journal of Clinical Oncology 2011;29(1):97‐105. [PUBMED: 21098317] - PMC - PubMed
Steensma 2011b {published data only}
-
- Steensma DP, Sloan JA, Dakhil SR, Dalton R, Kahanic SP, Prager DJ, et al. Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy‐associated anemia. Journal of Clinical Oncology 2011;29(1):97‐105. - PMC - PubMed
References to studies excluded from this review
Agrawal 2005 {published data only}
-
- Agrawal SG, Lim C, Cavill I. Prospective targeted epoietin beta therapy for chemotherapy‐associated anaemia achieves high response rates. Blood 2005;106:588.
Athibovonsuk 2013 {published data only}
-
- Athibovonsuk P, Manchana T, Sirisabya N. Prevention of blood transfusion with intravenous iron in gynecologic cancer patients receiving platinum‐based chemotherapy. Gynecologic oncology 2013;131(3):679‐82. [PUBMED: 24099839] - PubMed
Auerbach 2008 {published data only}
-
- Auerbach M, Silberstein PT, Webb T, Averyanova S, Ciuleanu T, Cam L, et al. Darbepoetin alfa (DA) 500mcg or 300mcg once every three weeks with or without iron in patients (pts) with chemotherapy‐induced anemia (CIA). Annals of Oncology 2008;19 (Suppl 8): viii:1‐4.
Birgegard 2006 {published data only}
-
- Birgegard G, Osterborg A, Hedenus M. Functional iron deficiency effectively overcome by adjuvant IV iron during epoetin treatment. ASH Annual Meeting Abstract 2006;108(11):3725.
Dangsuwan 2010 {published data only}
-
- Dangsuwan P, Manchana T. Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecologic Oncology 2010;116(3):522‐5. [PUBMED: 20051288] - PubMed
Demarteau 2007 {published data only}
-
- Demarteau N, Annemans L, Mossman T, Bracco A. Cost‐effectiveness of darbepoetin alfa (DA) 500 mcg every three weeks (Q3W) with IV iron compared to DA Q3W alone in cancer patients (pts) with chemotherapy‐induced anaemia (CIA). Journal of Clinical Oncology (Meeting Abstracts) 2007;25(18):Suppl 19531.
Doherty 2008 {published data only}
-
- Doherty EJ, Pappadakis J, Ryan K, Auerbach M. Intravenous iron improves efficacy and results in substantial savings in cost for patients receiving erythropoiesis‐stimulating agents (ESAs) for chemotherapy‐induced anemia. Journal of Clinical Oncology (Meeting Abstracts) May 2008;26(15):Suppl 20664.
Ferrari 2012 {published data only}
-
- Ferrari P, Nicolini A, Manca ML, Rossi G, Anselmi L, Conte M, et al. Treatment of mild non‐chemotherapy‐induced iron deficiency anemia in cancer patients: comparison between oral ferrous bisglycinate chelate and ferrous sulfate. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2012;66(6):414‐8. [PUBMED: 22795809] - PubMed
Hedenus 2007 {published data only}
-
- Hedenus M, Birgegard G, Nasman P, Ahlberg L, Karlsson T, Lauri B, et al. Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study. Leukemia 2007;21(4):627‐32. - PubMed
Hedenus 2014 {published data only}
-
- Hedenus M, Karlsson T, Ludwig H, Rzychon B, Felder M, Roubert B, et al. Intravenous iron alone resolves anemia in patients with functional iron deficiency and lymphoid malignancies undergoing chemotherapy. Medical oncology (Northwood, London, England) 2014;31(12):302. [PUBMED: 25373320] - PMC - PubMed
Kim 2007 {published data only}
-
- Kim YT, Kim SW, Yoon BS, Cho HJ, Nahm EJ, Kim SH, et al. Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. Gynecologic Oncology 2007;105(1):199‐204. [PUBMED: 17234260] - PubMed
Lerchenmueller 2006 {published data only}
-
- Lerchenmueller C, Husseini F, Gaede B, Mossman T, Suto T, Vanderbroek A. Intravenous (IV) iron supplementation in patients with chemotherapy‐induced anemia (CIA) receiving darbepoetin alfa every 3 weeks (Q3W): Iron parameters in a randomized controlled trial. Blood 2006;108(11):1552.
Maccio 2010 {published data only}
-
- Maccio A, Madeddu C, Gramignano G, Mulas C, Sanna E, Mantovani G. Efficacy and safety of oral lactoferrin supplementation in combination with rHuEPO‐beta for the treatment of anemia in advanced cancer patients undergoing chemotherapy: open‐label, randomized controlled study. The Oncologist 2010;15(8):894‐902. [PUBMED: 20647390] - PMC - PubMed
Pinter 2007 {published data only}
-
- Pinter T, Mossman T, Suto T, Vansteenkiste J. Effects of intravenous (IV) iron supplementation on responses to every‐3‐week (Q3W) darbepoetin alfa (DA) by baseline hemoglobin in patients (pts) with chemotherapy‐induced anemia (CIA). Journal of Clinical Oncology (Meeting Abstracts) June 20, 2007;25(18):9106.
Savonije 2006 {published data only}
-
- Savonije JH, Groeningen CJ, Broek WJ, Rentinck ME, Corstens FH, Gundy C, et al. Iron absorption during epoetin alfa therapy for chemotherapy‐associated anaemia. Cancer Investigation 2006;24(6):562‐6. [PUBMED: 16982459] - PubMed
Vandebroek 2006 {published data only}
-
- Vandebroek A, Gaede B, Altintas S, Smith K, Yao B, Schupp M, et al. A randomized open‐label study of darbepoetin alfa administered every 3 weeks with or without parenteral iron in anemic subjects with nonmyeloid malignancies receiving chemotherapy. Journal of Clinical Oncology (Meeting Abstracts) 2006;24(18):Suppl 8612.
References to ongoing studies
NCT01145638 {published data only}
-
- NCT01145638. A study of intravenous iron isomaltoside 1000 (Monofer®) as mono therapy (without erythropoiesis stimulating agents) in comparison with oral iron sulfate in subjects with non‐myeloid malignancies associated with chemotherapy induced anaemia (CIA). clinicaltrials.gov/show/NCT01145638 June 2010 (accessed July 2015).
Additional references
Aapro 2008
-
- Aapro MS, Link H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis‐stimulating agents. The Oncologist 2008;13(Suppl 3):33‐6. [PUBMED: 18458123] - PubMed
Auerbach 2008a
-
- Auerbach M. Should intravenous iron be the standard of care in oncology?. Journal of Clinical Oncology 2008;26(10):1579‐81. - PubMed
Bailie 2005
-
- Bailie GR, Clark JA, Lane CE, Lane PL. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrology Dialysis Transplantation 2005;20(7):1443‐9. [PUBMED: 15855210] - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines 3: Rating the quality of evidence ‐ introduction. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] - PubMed
Barrett‐Lee 2006
-
- Barrett‐Lee PJ, Ludwig H, Birgegard G, Bokemeyer C, Gascon P, Kosmidis PA, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology 2006;70(1):34‐48. [PUBMED: 16493206] - PubMed
Bennett 2008
-
- Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia. JAMA 2008;299(8):914‐24. [PUBMED: 18314434] - PubMed
Bohlius 2009
-
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis‐stimulating agents and mortality in patients with cancer: a meta‐analysis of randomised trials. The Lancet 2009;373(9674):1532‐42. - PubMed
Bohlius 2014
-
- Bohlius J, Tonia T, Nuesch E, Juni P, Fey MF, Egger M, et al. Effects of erythropoiesis‐stimulating agents on fatigue‐ and anaemia‐related symptoms in cancer patients: systematic review and meta‐analyses of published and unpublished data. British Journal of Cancer 2014;111(1):33‐45. [PUBMED: 24743705] - PMC - PubMed
Chertow 2004
-
- Chertow GM, Mason PD, Vaage‐Nilsen O, Ahlmen J. On the relative safety of parenteral iron formulations. Nephrology Dialysis Transplantation 2004;19(6):1571‐5. [PUBMED: 15150356] - PubMed
Chertow 2006
-
- Chertow GM, Mason PD, Vaage‐Nilsen O, Ahlmen J. Update on adverse drug events associated with parenteral iron. Nephrology Dialysis Transplantation 2006;21(2):378‐82. [PUBMED: 16286429] - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Demetri 1998
-
- Demetri GD, Kris M, Wade J, Degos L, Cella D. Quality‐of‐life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. Journal of Clinical Oncology 1998;16(10):3412‐25. [PUBMED: 9779721] - PubMed
Eschbach 2005
-
- Eschbach JW. Iron requirements in erythropoietin therapy. Best Practices Research Clinical Haematology 2005;18(2):347‐61. - PubMed
Fletes 2001
-
- Fletes R, Lazarus JM, Gage J, Chertow GM. Suspected iron dextran‐related adverse drug events in hemodialysis patients. American Journal of Kidney Diseases 2001;37(4):743‐9. [PUBMED: 11273874] - PubMed
Gabrilove 2007
-
- Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy‐induced anemia: results of a multicenter, open‐label study (SURPASS) of patients treated with darbepoetin‐alpha at a dose of 200 microg every 2 weeks. Cancer 2007;110(7):1629‐40. [PUBMED: 17694552] - PubMed
Gafter‐Gvili 2013
-
- Gafter‐Gvili A, Rozen‐Zvi B, Vidal L, Leibovici L, Vansteenkiste J, Gafter U, et al. Intravenous iron supplementation for the treatment of chemotherapy‐induced anaemia ‐ systematic review and meta‐analysis of randomised controlled trials. Acta Oncologica 2013;52(1):18‐29. [PUBMED: 22877242] - PubMed
Glaspy 1997
-
- Glaspy J, Bukowski R, Steinberg D, Taylor C, Tchekmedyian S, Vadhan‐Raj S. Impact of therapy with epoetin alfa on clinical outcomes in patients with nonmyeloid malignancies during cancer chemotherapy in community oncology practice. Procrit Study Group. Journal of Clinical Oncology 1997;15(3):1218‐34. - PubMed
Glaspy 2010
GRADEpro 2008 [Computer program]
-
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl E, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011a
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Guyatt 2011c
-
- Guyatt G, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ risk of bias. Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Henry 1995
-
- Henry DH, Brooks BJ Jr, Case DC Jr, Fishkin E, Jacobson R, Keller AM, et al. Recombinant human erythropoietin therapy for anemic cancer patients receiving cisplatin chemotherapy. The Cancer Journal From Scientific American 1995;1(4):252‐60. [PUBMED: 9166485] - PubMed
Higgins 2011a
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Khorana 2008
Kitano 2007
-
- Kitano T, Tada H, Nishimura T, Teramukai S, Kanai M, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. International Journal of Hematology 2007;86(1):37‐41. [PUBMED: 17675265] - PubMed
Knight 2004
-
- Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. The American Journal of Medicine 2004;116 Suppl 7A:11S‐26S. - PubMed
Leonard 2005
-
- Leonard RC, Untch M, Koch F. Management of anaemia in patients with breast cancer: role of epoetin. Annals of Oncology 2005;16(5):817‐24. - PubMed
Littlewood 2001
-
- Littlewood TJ, Bajetta E, Nortier JWR, Vercammen E, Rapoport B. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: Results of a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Oncology 2001;19(11):2865‐74. - PubMed
Ludwig 2004
-
- Ludwig H, Belle S, Barrett‐Lee P, Birgegard G, Bokemeyer C, Gascon P, et al. The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. European Journal of Cancer 2004;40(15):2293‐306. - PubMed
Mamula 2002
-
- Mamula P, Piccoli DA, Peck SN, Markowitz JE, Baldassano RN. Total dose intravenous infusion of iron dextran for iron‐deficiency anemia in children with inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition 2002;34(3):286‐90. - PubMed
Mancuso 2006
-
- Mancuso A, Migliorino M, Santis S, Saponiero A, Marinis F. Correlation between anemia and functional/cognitive capacity in elderly lung cancer patients treated with chemotherapy. Annals of Oncology 2006;17(1):146‐50. - PubMed
Mercadante 2009
-
- Mercadante S, Ferrera P, Villari P, David F, Giarratano A, Riina S. Effects of red blood cell transfusion on anemia‐related symptoms in patients with cancer. Journal of Palliative Medicine 2009;12(1):60‐3. - PubMed
Mhaskar 2012
-
- Mhaskar R, Wao H, Miladinovic B, Kumar A, Djulbegovic B. Role of iron supplementation to erythropoiesis stimulating agents in the management of chemotherapy‐induced anemia in cancer patients. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009624; CD009624] - DOI - PMC - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. - PubMed
NCCN 2009
-
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Cancer and treatment‐related anemia. http://www.nccn.org/professionals/physician_gls/PDF/anemia.pdf (accessed 18 June 2010).
NCCN 2010
-
- National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: Cancer and treatment‐related anemia. http://www.nccn.org/professionals/physician_gls/PDF/anemia.pdf (accessed 18 June 2010).
Orsini 2006
-
- Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose‐response data. Stata Journal 2006;6(1):40‐57.
Petrelli 2012
Pujade‐Lauraine
-
- Pujade‐Lauraine E, Gascon P. The burden of anaemia in patients with cancer. Oncology 2004;67 Suppl 1:1‐4. [PUBMED: 15489559] - PubMed
Razzouk 2006
-
- Razzouk BI, Hord JD, Hockenberry M, Hinds PS, Feusner J, Williams D, et al. Double‐blind, placebo‐controlled study of quality of life, hematologic end points, and safety of weekly epoetin alfa in children with cancer receiving myelosuppressive chemotherapy. Journal of Clinical Oncology 2006;24(22):3583‐9. - PubMed
RevMan 5.3 [Computer program]
-
- The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.3. Copenhagen: The Cochrane Collaboration, 2014.
Rizzo 2002
-
- Rizzo JD, Lichtin AE, Woolf SH, Seidenfeld J, Bennett CL, Cella D, et al. Use of epoetin in patients with cancer: Evidence‐based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. Journal of Clinical Oncology 2002;20(19):4083‐107. - PubMed
Rizzo 2008
-
- Rizzo JD, Somerfield MR, Hagerty KL, Seidenfeld J, Bohlius J, Bennett CL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. Journal of Clinical Oncology 2008;26(1):132‐49. [PUBMED: 17954713] - PubMed
Rizzo 2010
-
- Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Journal of Clinical Oncology 2010;28(32):4996‐5010. - PubMed
Schwartz 2007
-
- Schwartz RN. Anemia in patients with cancer: incidence, causes, impact, management, and use of treatment guidelines and protocols. American Journal of Health‐System Pharmacy 2007;64(3 Suppl 2):S5‐13. - PubMed
Shander 2010
-
- Shander A, Spence RK, Auerbach M. Can intravenous iron therapy meet the unmet needs created by the new restrictions on erythropoietic stimulating agents?. Transfusion 2010;50(3):719‐32. [PUBMED: 19919555] - PubMed
Shord 2008
-
- Shord SS, Hamilton JM Jr, Cuellar S. Parenteral iron with erythropoiesis‐stimulating agents for chemotherapy‐induced anemia. Journal of Oncology Pharmacy Practice 2008;14(1):5‐22. [PUBMED: 18337436] - PubMed
Stasi 2003
-
- Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer‐related fatigue: evolving concepts in evaluation and treatment. Cancer 2003;98(9):1786‐801. [PUBMED: 14584059] - PubMed
Stata 11 2009 [Computer program]
-
- StataCorp LP. Stata Statistical Software. Version 11. College Station, TX: StataCorp LP, 2009.
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [PUBMED: 12958120] - PubMed
Tierney 2007
Tonia 2012
van Weert 2006
-
- Weert E, Hoekstra‐Weebers J, Otter R, Postema K, Sanderman R, Schans C. Cancer‐related fatigue: predictors and effects of rehabilitation. The Oncologist 2006;11(2):184‐96. [PUBMED: 16476839] - PubMed
White 2009
-
- White IR, Higgins JP. Meta‐analysis with missing data. Stata Journal 2009;9(1):57.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

