Regulation of Stem Cell Proliferation and Cell Fate Specification by Wingless/Wnt Signaling Gradients Enriched at Adult Intestinal Compartment Boundaries
- PMID: 26845150
- PMCID: PMC4742051
- DOI: 10.1371/journal.pgen.1005822
Regulation of Stem Cell Proliferation and Cell Fate Specification by Wingless/Wnt Signaling Gradients Enriched at Adult Intestinal Compartment Boundaries
Abstract
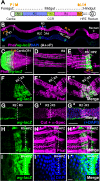
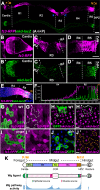
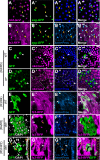
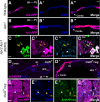
Intestinal stem cell (ISC) self-renewal and proliferation are directed by Wnt/β-catenin signaling in mammals, whereas aberrant Wnt pathway activation in ISCs triggers the development of human colorectal carcinoma. Herein, we have utilized the Drosophila midgut, a powerful model for ISC regulation, to elucidate the mechanisms by which Wingless (Wg)/Wnt regulates intestinal homeostasis and development. We provide evidence that the Wg signaling pathway, activation of which peaks at each of the major compartment boundaries of the adult intestine, has essential functions. Wg pathway activation in the intestinal epithelium is required not only to specify cell fate near compartment boundaries during development, but also to control ISC proliferation within compartments during homeostasis. Further, in contrast with the previous focus on Wg pathway activation within ISCs, we demonstrate that the primary mechanism by which Wg signaling regulates ISC proliferation during homeostasis is non-autonomous. Activation of the Wg pathway in absorptive enterocytes is required to suppress JAK-STAT signaling in neighboring ISCs, and thereby their proliferation. We conclude that Wg signaling gradients have essential roles during homeostasis and development of the adult intestine, non-autonomously controlling stem cell proliferation inside compartments, and autonomously specifying cell fate near compartment boundaries.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells.J Mol Cell Biol. 2010 Feb;2(1):37-49. doi: 10.1093/jmcb/mjp028. Epub 2009 Sep 30. J Mol Cell Biol. 2010. PMID: 19797317
-
Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells.Nature. 2008 Oct 23;455(7216):1119-23. doi: 10.1038/nature07329. Epub 2008 Sep 21. Nature. 2008. PMID: 18806781
-
The ADP-ribose polymerase Tankyrase regulates adult intestinal stem cell proliferation during homeostasis in Drosophila.Development. 2016 May 15;143(10):1710-20. doi: 10.1242/dev.127647. Development. 2016. PMID: 27190037 Free PMC article.
-
Intestinal stem cell response to injury: lessons from Drosophila.Cell Mol Life Sci. 2016 Sep;73(17):3337-49. doi: 10.1007/s00018-016-2235-9. Epub 2016 May 2. Cell Mol Life Sci. 2016. PMID: 27137186 Free PMC article. Review.
-
Powerful Drosophila screens that paved the wingless pathway.Fly (Austin). 2014;8(4):218-25. doi: 10.4161/19336934.2014.985988. Epub 2015 Jan 20. Fly (Austin). 2014. PMID: 25565425 Free PMC article. Review.
Cited by
-
Maintenance of the adult Drosophila intestine: all roads lead to homeostasis.Curr Opin Genet Dev. 2016 Oct;40:81-86. doi: 10.1016/j.gde.2016.06.009. Epub 2016 Jul 5. Curr Opin Genet Dev. 2016. PMID: 27392294 Free PMC article. Review.
-
Upregulated TNF/Eiger signaling mediates stem cell recovery and tissue homeostasis during nutrient resupply in Drosophila testis.Sci Rep. 2020 Jul 15;10(1):11674. doi: 10.1038/s41598-020-68313-7. Sci Rep. 2020. PMID: 32669615 Free PMC article.
-
The Intestine of Drosophila melanogaster: An Emerging Versatile Model System to Study Intestinal Epithelial Homeostasis and Host-Microbial Interactions in Humans.Microorganisms. 2019 Sep 9;7(9):336. doi: 10.3390/microorganisms7090336. Microorganisms. 2019. PMID: 31505811 Free PMC article. Review.
-
Wnt Signaling Rescues Amyloid Beta-Induced Gut Stem Cell Loss.Cells. 2022 Jan 14;11(2):281. doi: 10.3390/cells11020281. Cells. 2022. PMID: 35053396 Free PMC article.
-
dTcf/Pangolin suppresses growth and tumor formation in Drosophila.Proc Natl Acad Sci U S A. 2019 Jul 9;116(28):14055-14064. doi: 10.1073/pnas.1816981116. Epub 2019 Jun 24. Proc Natl Acad Sci U S A. 2019. PMID: 31235567 Free PMC article.
References
-
- Brunner E, Peter O, Schweizer L, Basler K (1997) pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 385: 829–833. - PubMed
-
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, et al. (1997) Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799. - PubMed
-
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, et al. (2002) Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109: 47–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

