Integration of endothelial protease-activated receptor-1 inflammatory signaling by ubiquitin
- PMID: 26845544
- PMCID: PMC4978167
- DOI: 10.1097/MOH.0000000000000232
Integration of endothelial protease-activated receptor-1 inflammatory signaling by ubiquitin
Abstract
Purpose of review: The maintenance and integrity of the endothelial barrier is essential for vascular homeostasis. Endothelial barrier dysfunction is mediated by various inflammatory factors, many of which act through G protein-coupled receptors including protease-activated receptors (PARs). PARs are expressed in multiple cell types in the vasculature and mediate cellular responses to thrombin, the key effector protease of the coagulation cascade. Thrombin activation of PAR1 induces endothelial barrier permeability through multiple pathways. Here, we discuss the mechanism by which thrombin activation of PAR1 promotes endothelial barrier breakdown and highlight recent advances that have provided new insight into molecular mechanisms that control endothelial barrier integrity.
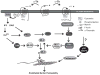
Recent findings: Although the signal transduction pathways induced by thrombin activation of PAR1 in endothelial cells have been extensively studied, the key regulatory mechanisms remain poorly understood. Posttranslational modifications are integral to the regulation of PAR1 signaling and recent studies suggest a novel function for ubiquitination of PAR1 in regulation of endothelial barrier permeability.
Summary: An understanding of how endothelial barrier permeability is regulated by thrombin activation of PAR1 is important for the discovery of new drug targets that can be manipulated to control endothelial barrier permeability and prevent progression of vascular inflammation.
Conflict of interest statement
The authors declare no conflicts of interest
Figures

Similar articles
-
A novel protein C-factor VII chimera provides new insights into the structural requirements for cytoprotective protease-activated receptor 1 signaling.J Thromb Haemost. 2017 Nov;15(11):2198-2207. doi: 10.1111/jth.13807. Epub 2017 Sep 21. J Thromb Haemost. 2017. PMID: 28834159
-
Phosphoproteomic analysis of protease-activated receptor-1 biased signaling reveals unique modulators of endothelial barrier function.Proc Natl Acad Sci U S A. 2020 Mar 3;117(9):5039-5048. doi: 10.1073/pnas.1917295117. Epub 2020 Feb 18. Proc Natl Acad Sci U S A. 2020. PMID: 32071217 Free PMC article.
-
Ubiquitin plays an atypical role in GPCR-induced p38 MAP kinase activation on endosomes.J Cell Biol. 2015 Sep 28;210(7):1117-31. doi: 10.1083/jcb.201504007. Epub 2015 Sep 21. J Cell Biol. 2015. PMID: 26391660 Free PMC article.
-
Neurocoagulation from a Mechanistic Point of View in the Central Nervous System.Semin Thromb Hemost. 2022 Apr;48(3):277-287. doi: 10.1055/s-0041-1741569. Epub 2022 Jan 20. Semin Thromb Hemost. 2022. PMID: 35052009 Review.
-
A New Concept of Action of Hemostatic Proteases on Inflammation, Neurotoxicity, and Tissue Regeneration.Biochemistry (Mosc). 2017 Jul;82(7):778-790. doi: 10.1134/S0006297917070033. Biochemistry (Mosc). 2017. PMID: 28918742 Review.
Cited by
-
Tranexamic acid blocks the thrombin-mediated delay of epidermal permeability barrier recovery induced by the cedar pollen allergen, Cry j1.Sci Rep. 2018 Oct 23;8(1):15610. doi: 10.1038/s41598-018-33898-7. Sci Rep. 2018. PMID: 30353092 Free PMC article.
-
Vasculopathy in COVID-19.Blood. 2022 Jul 21;140(3):222-235. doi: 10.1182/blood.2021012250. Blood. 2022. PMID: 34986238 Free PMC article. Review.
-
Rivaroxaban as a Protector of Oxidative Stress-Induced Vascular Endothelial Glycocalyx Damage via the IQGAP1/PAR1-2/PI3K/Akt Pathway.J Vasc Res. 2025;62(1):22-36. doi: 10.1159/000542419. Epub 2024 Nov 2. J Vasc Res. 2025. PMID: 39496251 Free PMC article.
-
Antagonizing the irreversible thrombomodulin-initiated proteolytic signaling alleviates age-related liver fibrosis via senescent cell killing.Cell Res. 2023 Jul;33(7):516-532. doi: 10.1038/s41422-023-00820-4. Epub 2023 May 11. Cell Res. 2023. PMID: 37169907 Free PMC article.
-
SARS-CoV-2 Mediated Endothelial Dysfunction: The Potential Role of Chronic Oxidative Stress.Front Physiol. 2021 Jan 15;11:605908. doi: 10.3389/fphys.2020.605908. eCollection 2020. Front Physiol. 2021. PMID: 33519510 Free PMC article. Review.
References
-
- O’Brien PJ, Prevost N, Molino M, Hollinger MK, Woolkalis MJ, Woulfe DS, et al. Thrombin Responses in Human Endothelial Cells. Contributions from receptors other than PAR1 include transactivation of PAR2 by thrombin-cleaved PAR1. J Biol Chem. 2000;275:13502–9. - PubMed
-
- Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

