Extracellular Vesicles from BOEC in In Vitro Embryo Development and Quality
- PMID: 26845570
- PMCID: PMC4742056
- DOI: 10.1371/journal.pone.0148083
Extracellular Vesicles from BOEC in In Vitro Embryo Development and Quality
Abstract
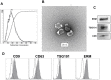
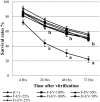
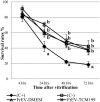
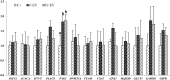
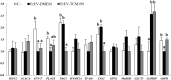
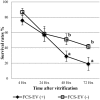
To evaluate the effect of conditioned media (CM) and Extracellular Vesicles (EVs) derived from bovine oviduct epithelial cell (BOEC) lines on the developmental capacity of bovine zygotes and the quality of embryos produced in vitro, presumptive zygotes were cultured under specific conditions. In experiment 1, zygotes were cultured either on monolayers from BOEC extended culture (E), together with fresh BOEC suspension cells, or with BOEC-CM from fresh or E-monolayers. In experiment 2, EVs were isolated from BOEC-CM and characterized (150-200 nm) by Nanosight® and electron microscopy. Zygotes were cultured in the presence of 3x10(5) EVs/mL, 1.5x10(5) EVs/mL or 7.5x10(4) EVs/mL of fresh or frozen BOEC-EVs. In experiment 3, zygotes were cultured in absence of FCS but with EVs from BOEC-E that had been cultured in different culture media. In experiment 4, zygotes were cultured in SOF+5% normal-FCS, or EV-depleted-FCS. In all cases, cleavage rate (Day 2) and blastocyst development (Day 7-9) was assessed. Blastocysts on Days 7/8 were used for quality evaluation through differential cell count, cryotolerance and gene expression patterns. No differences were found among all FCS-containing groups in cleavage rate or blastocyst yield. However, embryos derived from BOEC-CM had more trophectoderm cells, while embryos derived from BOEC-EVs, both fresh and frozen, has more trophectoderm and total cells. More embryos survived vitrification in the BOEC-CM and BOEC-EV groups. In contrast, more embryos survived in the EV-depleted-FCS than in normal-FCS group. Gene expression patterns were modified for PAG1 for embryos cultured with EVs in the presence of FCS and for IFN-T, PLAC8, PAG1, CX43, and GAPDH in the absence of FCS. In conclusion, EVs from FCS have a deleterious effect on embryo quality. BOEC-CM and EVs during in vitro culture had a positive effect on the quality of in vitro produced bovine embryos, suggesting that EVs have functional communication between the oviduct and the embryo in the early stages of development.
Conflict of interest statement
Figures

References
-
- Niemann H, Wrenzycki C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology. 2000;53: 21–34. - PubMed
-
- Pontes JHF, Nonato-Junior I, Sanches BV, Ereno-Junior JC, Uvo S, Barreiros TRR, et al. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology. 2009;71: 690–697. 10.1016/j.theriogenology.2008.09.031 - DOI - PubMed
-
- Hackett AJ, Durnford R, Mapletoft RJ, Marcus GJ. Location and status of embryos in the genital tract of superovulated cows 4 to 6 days after insemination. Theriogenology. 1993;40: 1147–1153. 10.1016/0093-691X(93)90285-D - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

