Insulinoma After Bariatric Surgery: Diagnostic Dilemma and Therapeutic Approaches
- PMID: 26846121
- PMCID: PMC4814298
- DOI: 10.1007/s11695-016-2092-5
Insulinoma After Bariatric Surgery: Diagnostic Dilemma and Therapeutic Approaches
Abstract
Hypoglycemia is increasingly recognized as a complication of bariatric surgery. Typically, hypoglycemia does not appear immediately postoperatively, but rather more than 1 year later, and usually occurs 1-3 h after meals. While rare, insulinoma has been reported after bariatric surgery. Clinical factors which should raise suspicion for insulinoma and the need for comprehensive clinical and biochemical evaluation include hypoglycemia occurring in the fasting state, predating bariatric surgery, and/or worsening immediately postoperatively, and lack of response to conservative therapy. Localization and successful resection of insulinoma can be achieved using novel endoscopic ultrasound and surgical approaches. In summary, hypoglycemia presenting shortly after gastric bypass or with a dominant fasting pattern should be fully evaluated to exclude insulinoma. Additionally, evaluation prior to gastric bypass should include screening for history of hypoglycemia symptoms.
Keywords: Bariatric surgery; Gastric bypass; Hypoglycemia; Insulinoma; Minimally invasive surgery.
Conflict of interest statement
Authors CMM, AS, EUY, DL, MSS, and AJM declare no conflict of interest. MEP reports grants from American Society for Metabolic and Bariatric Surgery, Medimmune, Nuclea Biosciences, Bristol-Myers Squibb, Astra-Zeneca, Novo-Nordisk Foundation, and Sanofi outside the submitted work. In addition, Dr. Patti has a submitted patent “Methods and Compositions for Treating Hypoglycemia.”
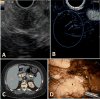
Figures



References
-
- Papamargaritis D, et al. Dumping symptoms and incidence of hypoglycaemia after provocation test at 6 and 12 months after laparoscopic sleeve gastrectomy. Obes Surg. 2012;22(10):1600–1606. - PubMed
-
- Goldfine AB, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678–4685. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

