Significance of the urokinase-type plasminogen activator and its receptor in the progression of focal segmental glomerulosclerosis in clinical and mouse models
- PMID: 26846181
- PMCID: PMC4743092
- DOI: 10.1186/s12929-016-0242-7
Significance of the urokinase-type plasminogen activator and its receptor in the progression of focal segmental glomerulosclerosis in clinical and mouse models
Erratum in
-
Erratum to: Significance of the urokinase-type plasminogen activator and its receptor in the progression of focal segmental glomerulosclerosis in clinical and mouse models.J Biomed Sci. 2017 Jul 19;24(1):48. doi: 10.1186/s12929-017-0348-6. J Biomed Sci. 2017. PMID: 28724378 Free PMC article. No abstract available.
Abstract
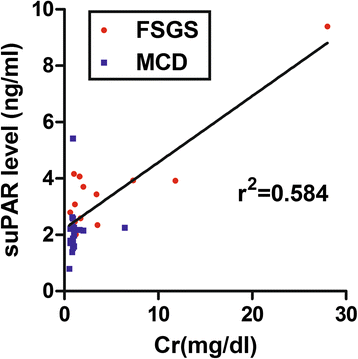
Background: suPAR biomarker generally considered a pathogenic factor in FSGS. However, studies have been published that dispute this conclusion. The current study was designed to investigate the roles of uPA and suPAR in FSGS in clinical and mouse models.
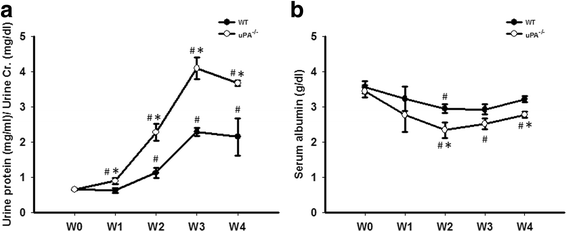
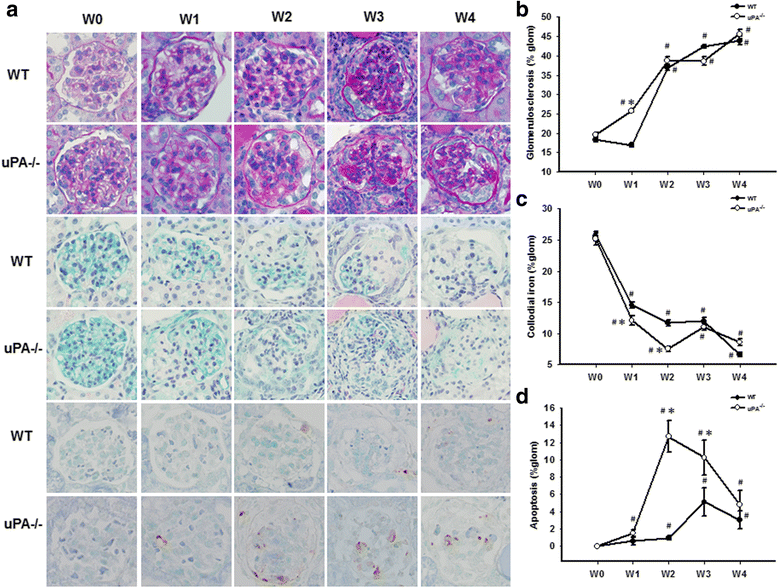
Methods: Clinical subjects including those with biopsy-proven FSGS and MCD were enrolled. To verify the role of uPA in FSGS, Adriamycin was used to induce FSGS in uPA knockout (uPA(-/-)) and BALB/c (WT) mice. Proteinuria and suPAR, the cleaved/intact forms of the circulating suPAR, and possible proteases involving cleavage of the suPAR were also studied.
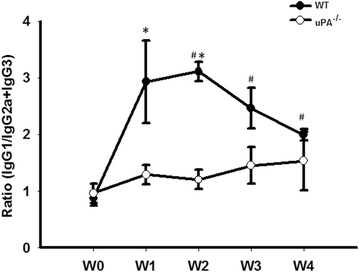
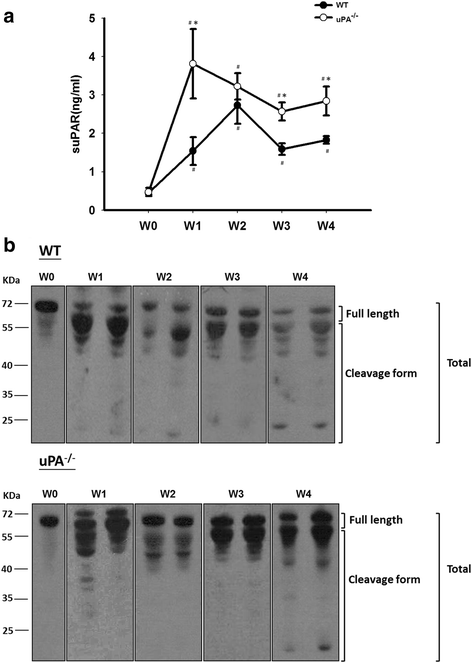
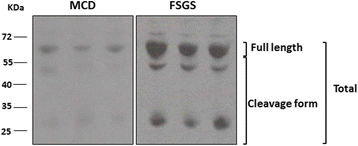
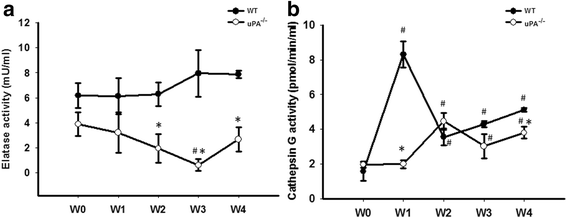
Results: FSGS clinical cases presented significantly higher serum levels of suPAR and Cr and lower serum levels of uPA. In the mice model, the uPA(-/-) group exhibited faster disease progression and worsening proteinuria than the WT group. In addition, the uPA(-/-) group had higher plasma suPAR levels, glomerular cell apoptosis, and dysregulation of the Th1/Th2 balance. In an analysis of suPAR variants in FSGS, both the intact and cleaved forms of the suPAR were higher in clinical subjects and the mouse model. However, the process of suPAR cleavage was not mediated by enzymatic activities of the uPA, elastase, or cathepsin G.
Conclusions: A deficiency of uPA accelerated the progression of Adriamycin-induced mouse FSGS model. Decrease of serum uPA levels may be an indicator of the progression of FSGS in clinical subjects and animal models.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

